A kind of method for preparing diazoxide
A technology of diazoxide and dimethylacetamide, applied in the field of compound preparation, can solve the problems of toxicity, easy moisture absorption, long steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The method for preparing diazoxide disclosed by the present invention is as follows:
[0033] Mix 2-amino-5-chlorobenzenesulfonamide, imidazolium salt and amide solvent and react with heating to obtain diazoxide. The preparation method of further 2-amino-5-chlorobenzenesulfonamide is as follows, anthranilamide and N - Chlorosuccinimide is reacted in a solvent to give 2-amino-5-chlorobenzenesulfonamide.
[0034] or
[0035] Mix anthranilamide, imidazolium salt and amide solvent and heat to react to obtain compound IV; then compound IV and N - Chlorosuccinimide is reacted in a chlorine solvent to give diazoxide.
[0036]
[0037] The column chromatography purification of all embodiments adopts ethyl acetate / petroleum ether with a volume ratio of 1:1 as eluent, R f is 0.3.
Embodiment 1
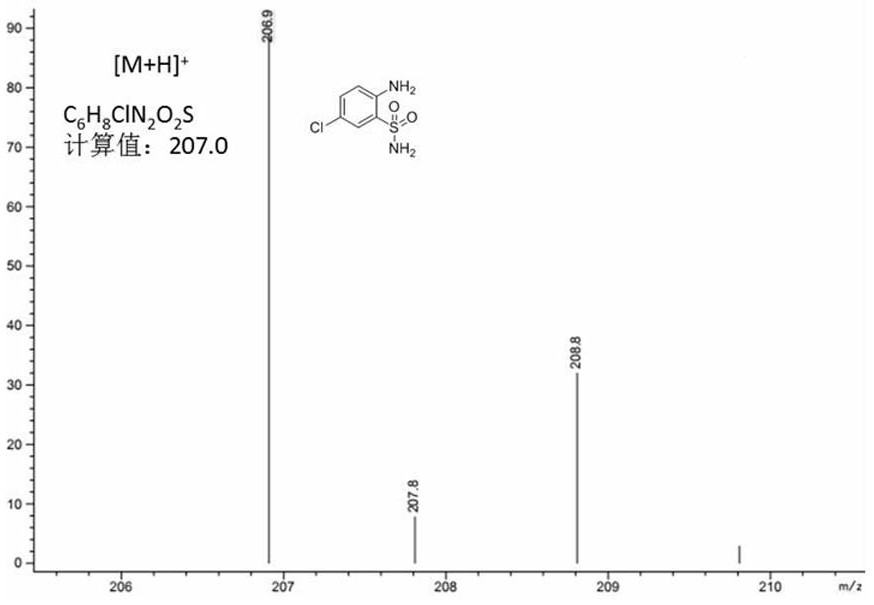
[0039] Anthranilamide (1.72g) was mixed with NCS (1.32g, 1.0 equivalent), dissolved in dichloromethane, stirred at reflux temperature for 5 hours, and then purified by column chromatography to prepare compound II 2-amino -5-chlorobenzenesulfonamide, 1.75g, the yield of this step is 85%, and the purity is greater than 99%. Compound Mass Spectrum: Calculated [M+H] + C 6 h 8 ClN 2 o 2 S, 206.99; experimental value: 206.83.
[0040] Anthranilamide (1.72g) was mixed with NCS (1.32g, 1.0 equivalent), dissolved in chloroform, stirred at reflux temperature for 3 hours, and then purified by column chromatography to prepare compound II2-amino-5- Chlorobenzenesulfonamide 1.96g, the yield of this step is 95%, the purity is greater than 99%, the experimental value of mass spectrum: 206.9, see the mass spectrum figure 1 .
Embodiment 2
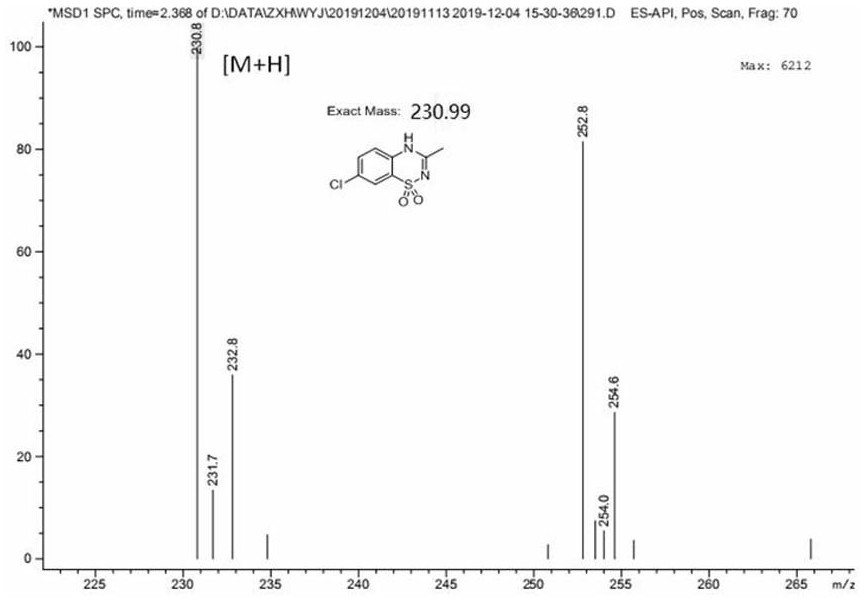
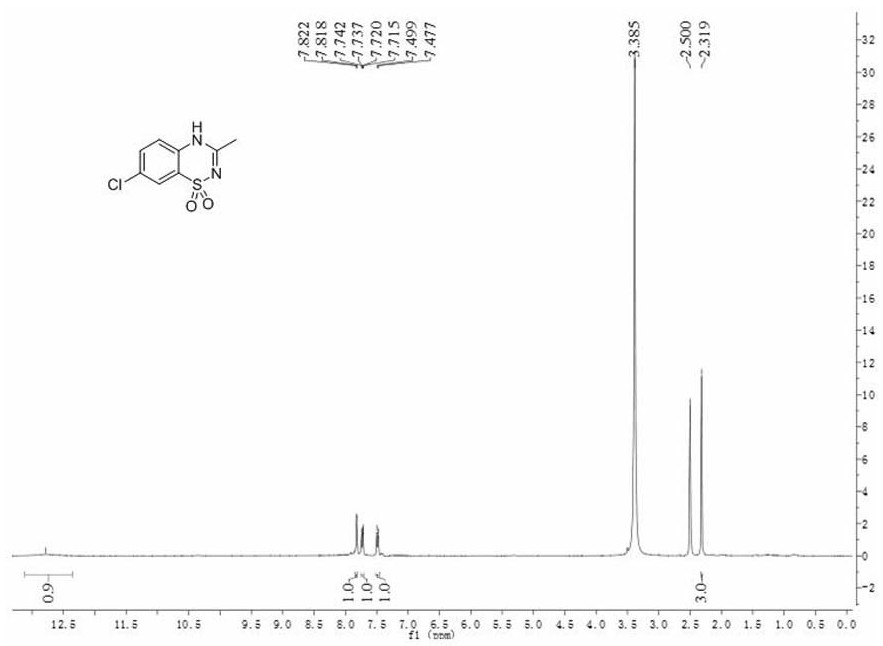
[0042] Dissolve 2-amino-5-chlorobenzenesulfonamide (2.05g) in 8.0ml N , N -In dimethylacetamide, add 0.14g (10mol%) imidazole hydrochloride again, reaction temperature 120 o C, after stirring for 48 hours, the reaction solution was distilled, and the excess N , N - Dimethylacetamide was recovered by distillation, and the residue was purified by column chromatography to obtain 2.07 g of the product diazoxide (compound III), with a yield of 90%, a purity greater than 99%, and an experimental value of mass spectrometry: 230.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com