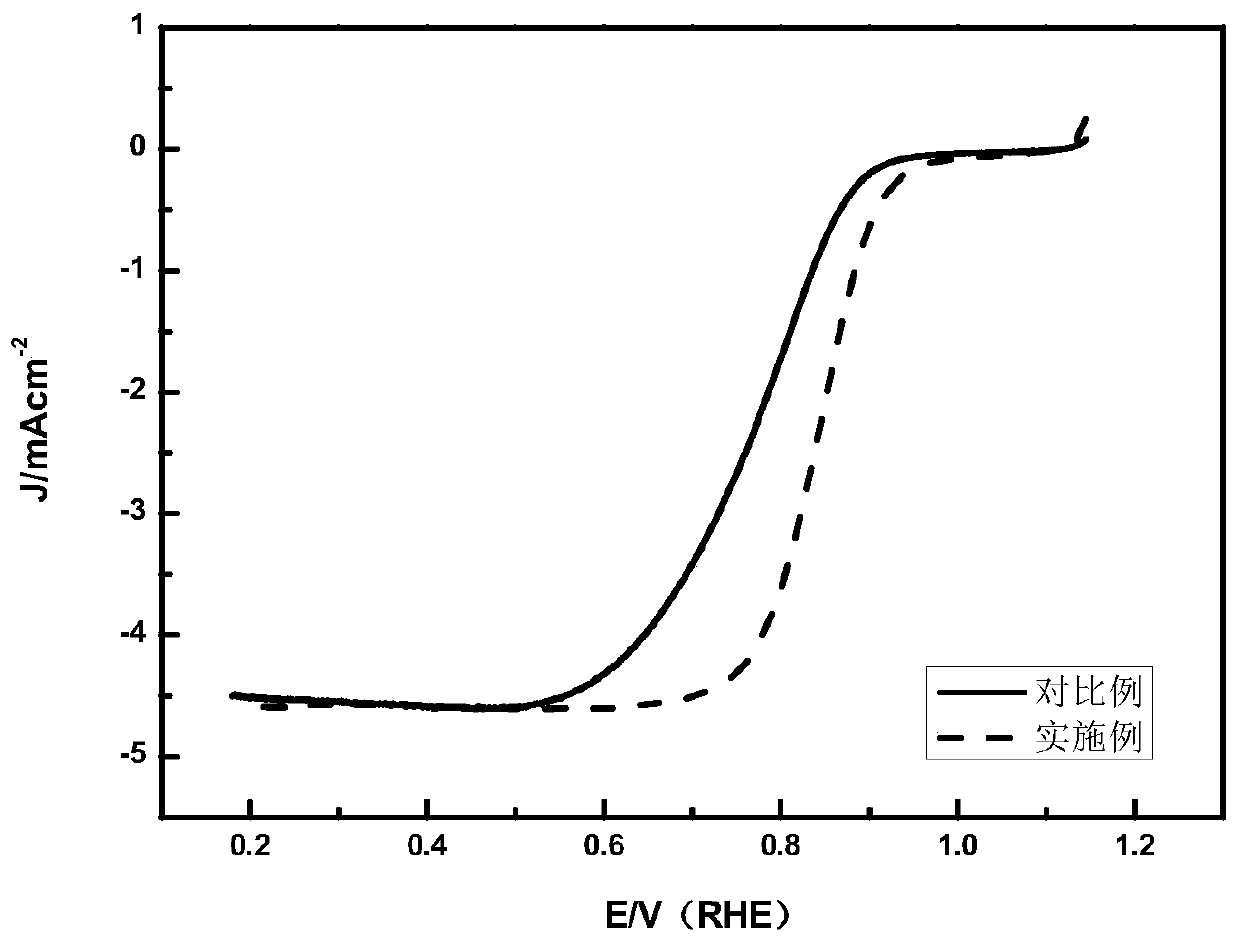
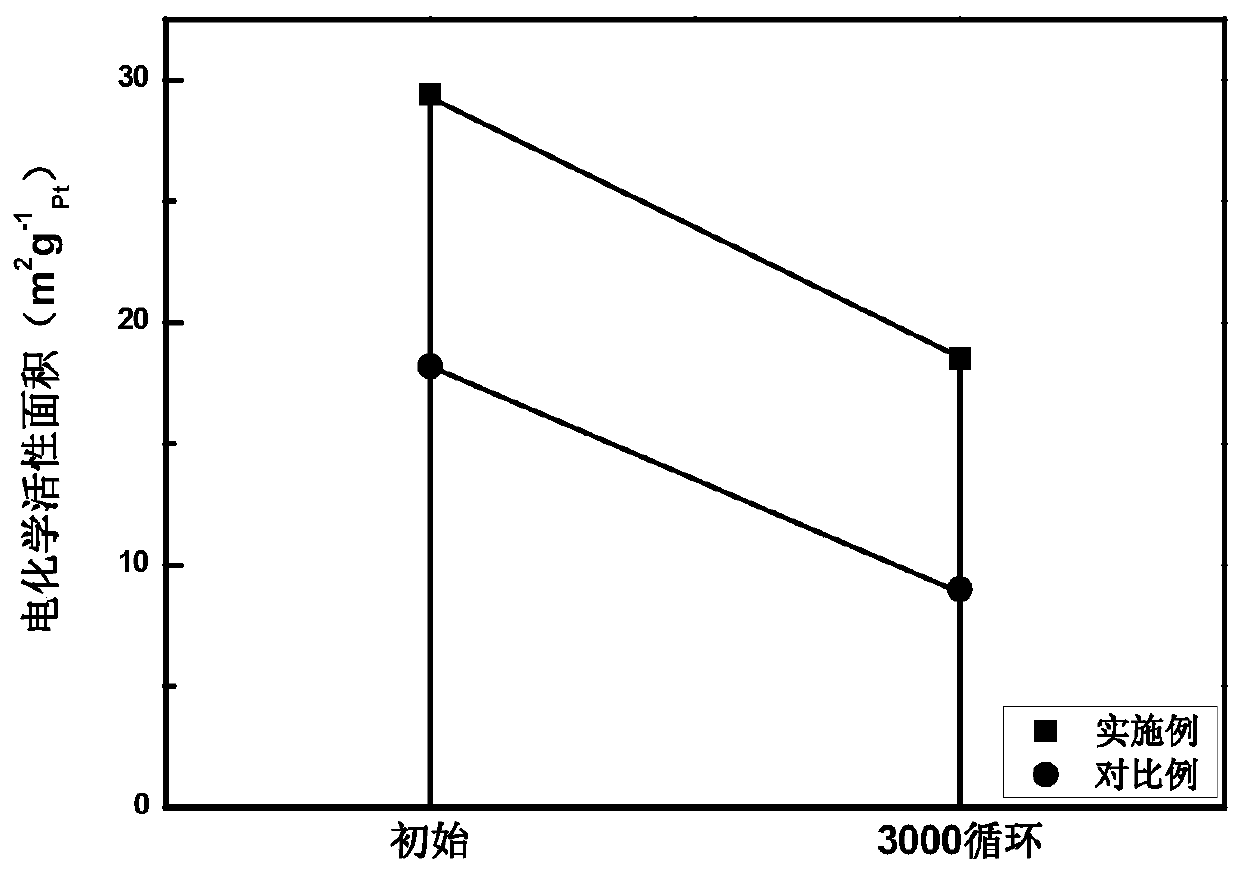
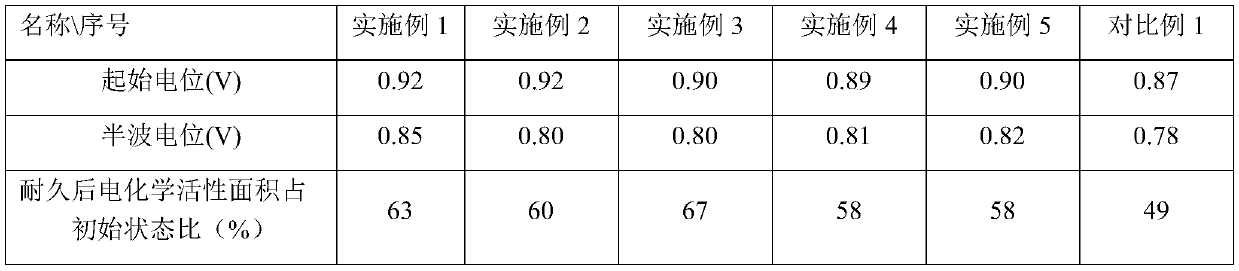
Carbon-supported noble metal alloy catalyst as well as preparation method and application thereof
A technology of alloy catalysts and noble metals, which is applied in the synthesis of fuel cell cathode catalysts, can solve the problems of transition metals easy to migrate and fall off, reduce the catalytic effect of oxygen reduction, and insufficient binding force, so as to achieve good catalytic performance of oxygen reduction and increase porosity and specific surface area, the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] 1. Put 8g of glucose into a 100ml beaker, add deionized water to make a 60ml solution, stir for 20min, add 10ml of aqueous solution containing 1g of nickel chloride hexahydrate dropwise while stirring, then add dropwise in sodium bicarbonate solution to adjust to pH= 8.
[0081] 2. Transfer the above solution into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction at 160°C for 6 hours.
[0082] 3. After the liquid is naturally cooled, centrifuge and wash the solid repeatedly with deionized water and ethanol until the filtrate is clear.
[0083] 4. Put the washed solid in a vacuum drying oven at 80° C. for 6 hours to obtain a dried carbon-loaded powder with a template.
[0084] 5. The above powder was placed in an atmosphere of 10% vol hydrogen and 90 vol% nitrogen, and the temperature was programmed to rise to 500° C. for 3 hours, and then cooled to room temperature to obtain carbon-supported nickel.
[0085] 6. Take 0.2g car...
Embodiment 2
[0090] 1. Put 5g of fructose into a 100ml beaker, add deionized water to make a 60ml solution, stir for 10min, add 10ml of an aqueous solution containing 0.9g of cobalt nitrate hexahydrate dropwise while stirring, then add ammonia solution dropwise to adjust to pH=9.
[0091] 2. Transfer the above solution into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction at 180°C for 5 hours.
[0092] 3. After the liquid is naturally cooled, centrifuge and wash the solid repeatedly with deionized water and ethanol until the filtrate is clear.
[0093] 4. Dry the washed solid in a vacuum oven at 90°C for 6 hours to obtain a carbon-loaded powder with a template.
[0094] 5. The above powder was placed in an atmosphere of 20vol% hydrogen and 80vol% argon, and the temperature was programmed to rise to 500° C. for 3 hours, and then cooled to room temperature to obtain carbon-supported cobalt.
[0095] 6. Take 0.14g of carbon-supported cobalt and ...
Embodiment 3
[0100] 1. Put 4g of starch into a 100ml beaker, add deionized water to make a 50ml solution, stir for 20min, add 10ml of an aqueous solution containing 0.5g of ferric chloride dropwise while stirring, then add acetic acid dropwise to adjust to pH=5.
[0101] 2. Transfer the above solution into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction at 170° C. for 12 hours.
[0102] 3. After the liquid is naturally cooled, centrifuge and wash the solid repeatedly with deionized water and ethanol until the filtrate is clear.
[0103] 4. Dry the washed solid in a vacuum oven at 80°C for 8 hours to obtain a carbon-loaded powder with a template.
[0104] 5. Place the catalyst precursor above in an atmosphere of 5 vol% hydrogen and 95 vol% helium, and program the temperature to 700° C. for 1 hour, and then cool to room temperature to obtain carbon-supported iron.
[0105] 6. Prepare 70ml solution of 0.22g carbon-supported iron with deionized ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap