Composition for upper respiratory tract infection and application thereof
A technology of upper respiratory tract and composition, applied in the field of composition of upper respiratory tract infection, can solve the problems of affecting work and life, accompanied by serious complications, high morbidity and infectivity, etc., and achieve the effect of good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of chlorogenic acid and artemisinone composition
[0021] Composition 1: Accurately weigh 0.333g of chlorogenic acid and 0.667g of artemisinone, add 10ml of physiological saline and mix well, and pass through a 0.22μm filter membrane to sterilize.
[0022] Composition 2: Accurately weigh 0.5g of chlorogenic acid and 0.5g of artemisinone, add 10ml of normal saline and mix well, pass through a 0.22μm filter membrane to sterilize it.
[0023] Composition 3: Accurately weigh 0.667g of chlorogenic acid and 0.333g of artemisinone, add 10ml of physiological saline and mix well, and pass through a 0.22μm filter membrane to sterilize.
Embodiment 2
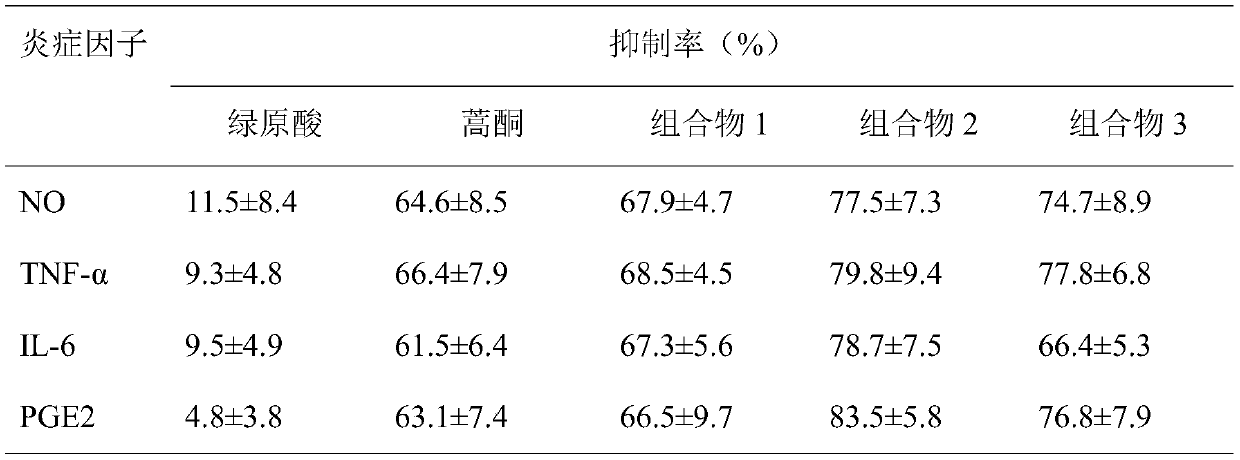
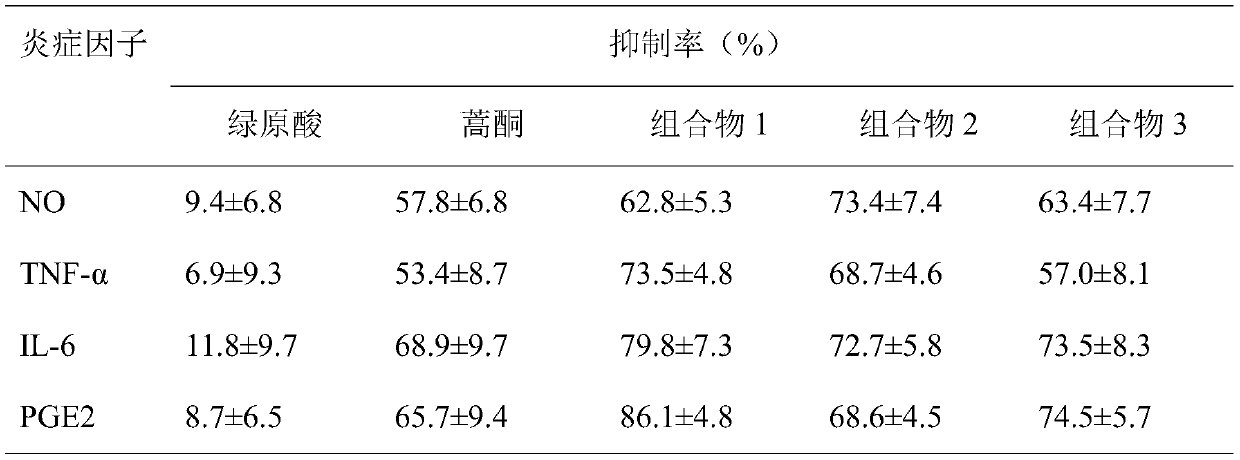
[0024] Example 2 Anti-inflammatory activity of composition
[0025] 1 Experimental materials
[0026] 1.1 cell line
[0027] The mouse macrophage cell line RAW 264.7 was purchased from Shanghai Cell Bank of Chinese Academy of Sciences.
[0028] 1.2 Drugs and reagents
[0029] DMEM / HIGH GLUCOSE, Gibco;
[0030] FBS: Gibco,
[0031] LPS: sigma;
[0032] Poly I:C:Sigma
[0033] DMSO: sigma;
[0034] 0.25% Trypsin-EDTA: Gibco
[0035] NO detection kit: Biyuntian, product number: S0021;
[0036] Mouse IL-6 detection kit: invitrogen, Lot:1913800020;
[0037] Mouse TNF-α detection kit: invitrogen, Lot: 184786017;
[0038] Mouse PGE2 detection kit: Enzo Life Sciences, Lot: 08211709C.
[0039] 1.3 Experimental equipment
[0040] CO 2 Cell incubator: thermo company;
[0041] Nikon TS100 inverted microscope: Nikon;
[0042] Ultra-clean workbench: Suzhou Aklin Purification Equipment Co., Ltd.;
[0043] Microplate reader: Molecular Devices;
[0044] Pipette: Eppendorf;
[0045] Centrifuge: Hunan Xiangyi Laboratory ...
Embodiment 3
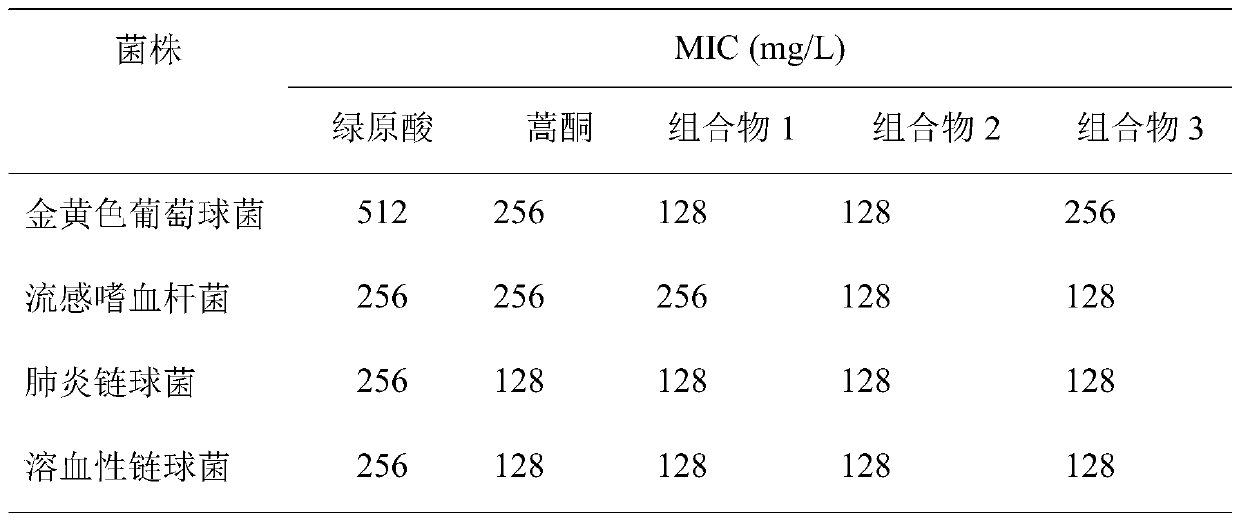
[0065] Example 3 Antibacterial activity of the composition
[0066] 1. Experimental materials
[0067] 1.1 strain
[0068] Streptococcus hemolyticus ATCC32210
[0069] Haemophilus influenzae, ATCC49247
[0070] Streptococcus pneumoniae ATCC49619
[0071] Staphylococcus aureus ATCC25923
[0072] 1.2 Drugs and reagents
[0073] Nutrient broth medium for the cultivation of Staphylococcus aureus
[0074] Brain Heart Infusion Broth (BHI) medium for the cultivation of Haemophilus influenzae
[0075] Mueller-Hinton medium for culture of hemolytic streptococcus and streptococcus pneumoniae
[0076] 1.3 Experimental equipment
[0077] CO 2 Cell incubator: thermo company;
[0078] Ultra-clean workbench: Suzhou Aklin Purification Equipment Co., Ltd.;
[0079] Microplate reader: Molecular Devices;
[0080] Pipette: Eppendorf;
[0081] 2 experimental steps
[0082] 2.1 Minimum inhibitory concentration detection
[0083] Measured by microdilution method. Scrape the fresh colonies grown on the plate with a cotton...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap