Use of a triphenylamine-modified binaphthyl derivative
A technology of binaphthyl derivatives and triphenylamine, which is applied in the field of triphenylamine modified binaphthyl derivatives, and can solve the problems of spectral changes, detection of iron ions without relevant research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 compound
[0052] (1) Synthesis of Compound 1
[0053] The synthetic route of compound 1 is as follows:
[0054]
[0055] The specific synthetic steps of compound 1 are as follows:
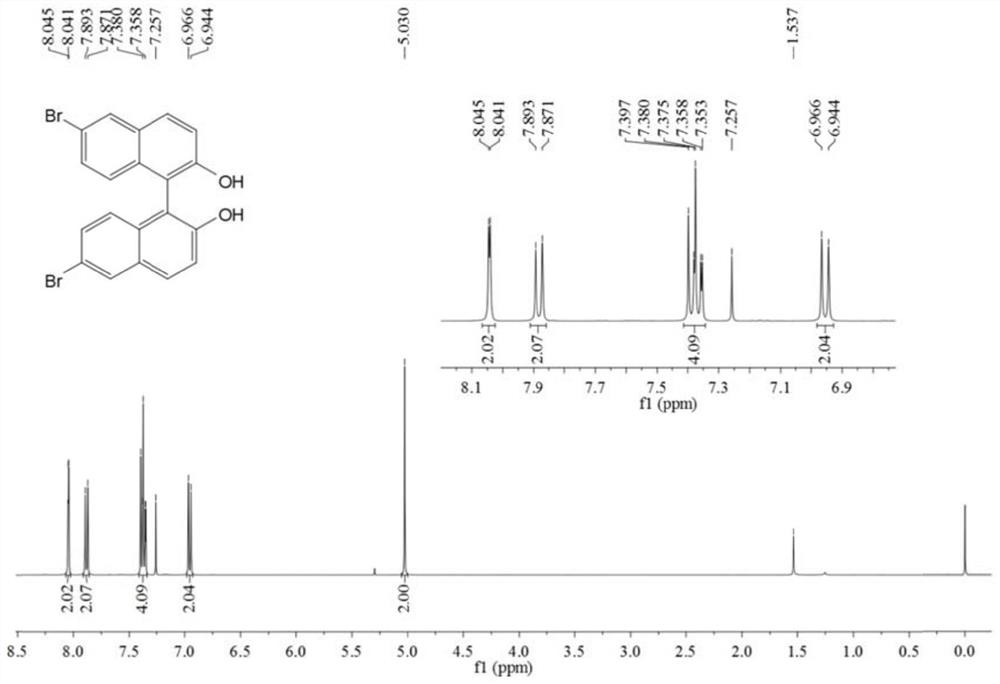
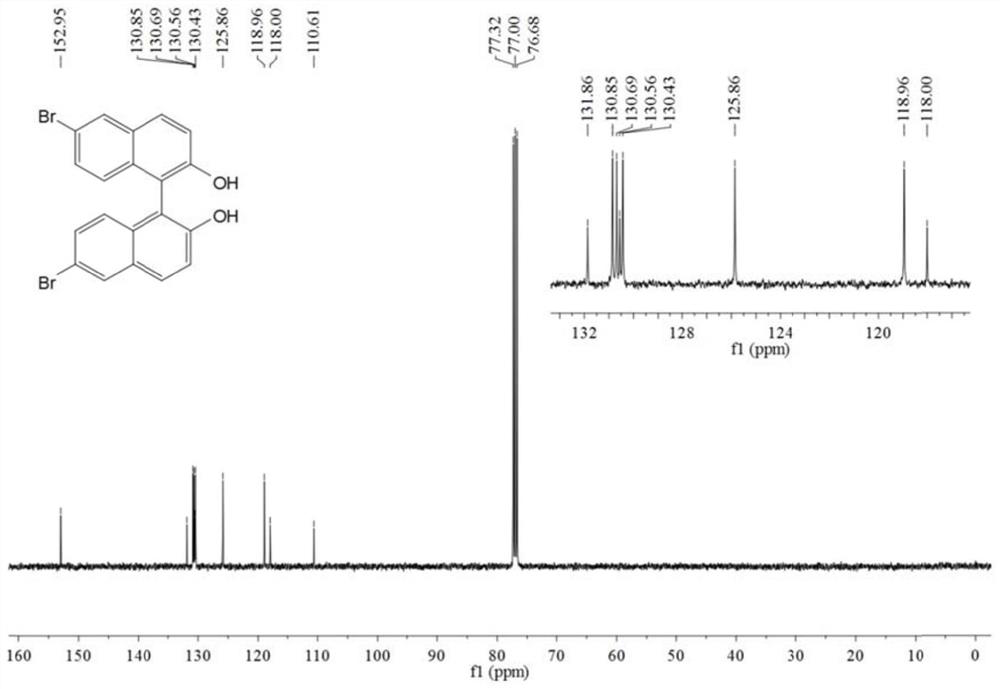
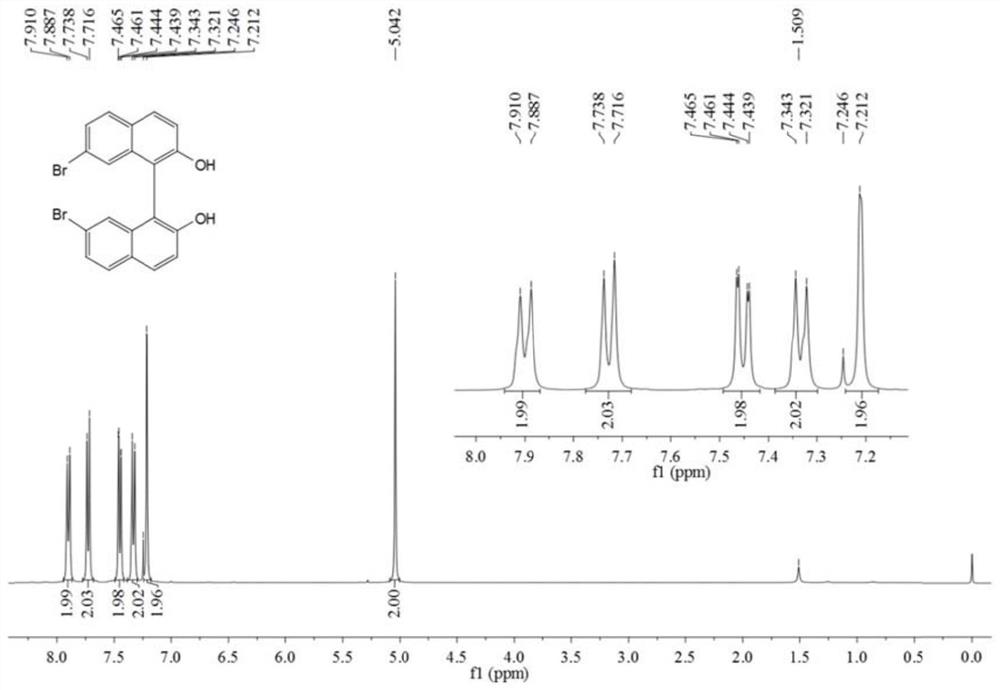
[0056] In a 250mL round bottom flask, add 6-bromo-2-naphthol (5g, 22.4mmol), FeCl 3 ·6H 2 O (12.1 g, 44.8 mmol) and distilled water (100 mL). After stirring the reaction at 60 °C for 24 h, it was cooled to room temperature. Dichloromethane (DCM) was added to the reaction liquid, fully dissolved and extracted and separated to obtain an organic phase. The obtained organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and distill under reduced pressure to obtain the crude product. The crude product was further purified by silica gel column chromatography. The eluent system was n-hexane and ethyl acetate (6:1) to obtain a white solid with a yield of 74.3% (3.7 g, 8.3 mmol).
[0057] The obtained white solid was characterized by a nuclear magn...
Embodiment 2
[0093] Embodiment 2 Iron ion titration experiment and detection limit calculation
[0094] Prepare 250mL 2×10 with a volumetric flask -5 The THF solution of M probe, take 10mL solution accurately with a pipette each time, add 20uL 0-40equiv of FeCl during stirring 3 aqueous solution, continue to stir for 3min to make the probe and Fe 3+ After sufficient exposure, measure the fluorescence emission spectrum of the corresponding solution.
[0095] Such as Figure 15 As shown, adding Fe 3+ It can weaken the fluorescence intensity until the fluorescence is quenched, and different Fe can be observed with the naked eye under a 365nm ultraviolet lamp 3+ Fluorescence response of the probe solution with different concentration, and take pictures and record in time. Among them, in the THF solution of 6,6-TB-1, with the addition of Fe 3+ The fluorescence intensity decreased sharply with a slight red shift as the amount increased from 0 to 40 equiv. while for 7,7-OMeTB-1, 15 equival...
Embodiment 3
[0097] Embodiment 3 selectivity test experiment
[0098] To evaluate the selectivity of the probe, we tested the fluorescence response of the probe with various metal ions. Prepare 250mL 2×10 with a volumetric flask -5 For the THF solution of the M probe, accurately take 10 mL of the solution with a pipette each time, and add 15 equivalents or 40 equivalents of different metal ions (Fe 3+ , K + , Mg 2+ , Na + , Ni 2+ ,Co 2+ , Ca 2+ , Al 3+ , Ba 2+ , Mn 2+ , Cr 3+ , Cu 2 + , Cd 2+ , Pd 2+ and Zn 2+ ), and measure the fluorescence emission spectrum of the corresponding solution after fully stirring. Under the 365nm ultraviolet lamp, observe and take pictures in time to record the fluorescence response ( Figure 17 ). In order to more intuitively observe the effects of various metal ions on the fluorescence intensity of the solution, we use F / F 0 is the ordinate (F is the fluorescence intensity of the solution after adding each metal ion, F 0 For the fluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com