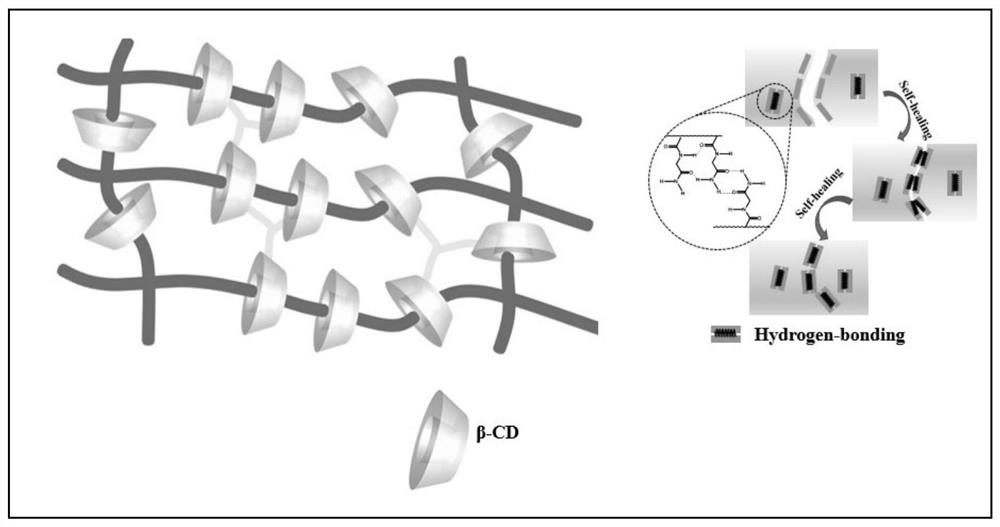
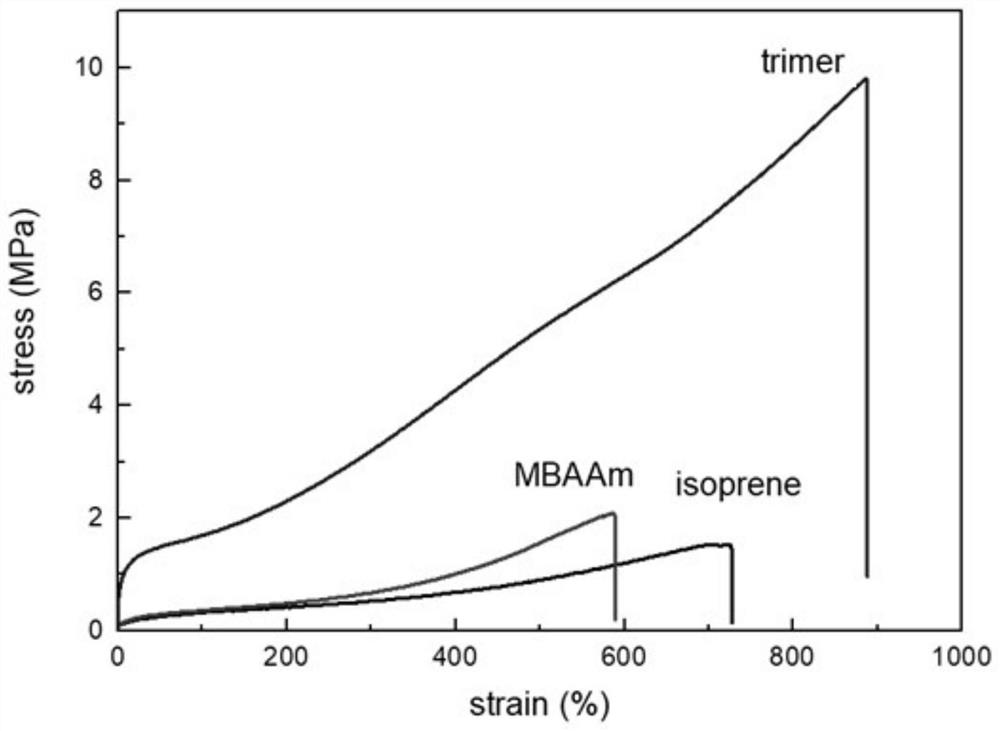
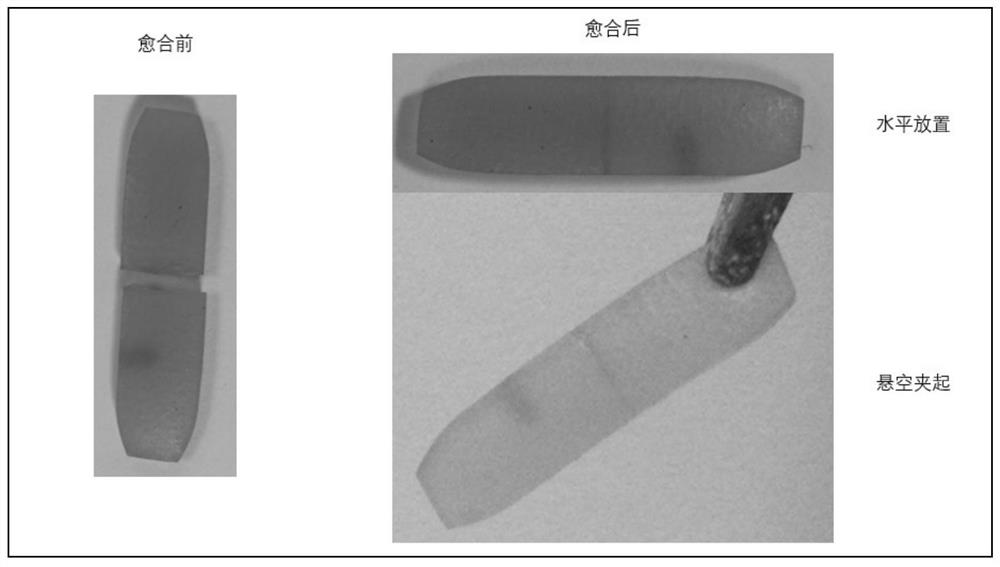
A self-healing elastomer with high cross-linking topology and its preparation method and application
An elastic body and self-healing technology, applied in the direction of insulators, plastic/resin/wax insulators, organic insulators, etc., can solve the problem of not being able to improve mechanical strength and stretchability at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment is the preparation of self-healing elastomer, specifically as follows:
[0044] (1) Synthesis of triynes: 1,3,5-benzenetricarboxylic acid (15.0g, 72mmol) was dissolved in thionyl chloride (45ml, 0.63mol, excess), and a few drops of DMF were added dropwise, and the reaction mixture was refluxed 8h, converted to 1,3,5-benzenetricarboxylic acid chloride (pale yellow liquid); then at 0°C, the obtained trichloride (1.06g, 4.0mol) was dissolved in CH 2 Cl 2 , and triethylamine (2.8ml, 20mmol, catalyst) was added, then the mixture was added dropwise to propargylamine (0.8ml, 12.6mmol) in CH 2 Cl 2 solution, after stirring at room temperature for 24 h, the resulting mixture was concentrated, dissolved in ethyl acetate (200-300 ml), and successively washed with 1M H 2 SO 4 , water, saturated NaHCO 3 Wash the organic layer with anhydrous Na 2 SO 4 Dry, filter and spin dry to obtain the tripyne compound.
[0045] (2) Synthesis of azide monosubstituted β-cyc...
Embodiment 2
[0055] This embodiment is the preparation of self-healing elastomer, specifically as follows:
[0056] (1) Preparation of Alkyne-Modified Mono(6-ethylenediamino)-β-cyclodextrin (Alkyne-Modified EDA-β-CD)
[0057] Synthesis of 4-oxo-4-(prop-2-ynyloxy)butanoic acid: propargyl alcohol (4.76g, 0.085mol), succinic anhydride (10.6g, 0.116mol), pyridine (9.18g, 0.116mol ) and TEA (8.46 g, 0.116 mol) were dissolved in 100 mL of anhydrous 1,4-dioxane. The mixture was stirred at room temperature for 24 hours, then evaporated under vacuum. Dissolve the crude product in CH 2 Cl 2 and washed with cold 1M HCl. The organic phase was washed with MgSO 4 Dry, filter and evaporate. The product was isolated as a brown solid.
[0058] Take 5g of Tos-β-CD in 30ml of ethylenediamine, and heat the reaction at 100°C for 12h. After the reaction is complete, cool to room temperature, add excess acetone to precipitate, and filter to obtain a light yellow solid. Then, the obtained light yellow so...
Embodiment 3
[0064] This embodiment is the preparation of self-healing elastomer, specifically as follows:
[0065] (1) Modification of guest molecules: cholic acid has multiple functional groups that can be modified, and can be easily introduced into the main chain polymer. The head and the tail of cholic acid are respectively modified with amino functional groups to obtain water-soluble cholic acid derivatives (cholic acid diamine). First, cholic acid is modified into 3-aminomethyl cholate with higher activity, and then After dissolving in dry ethylenediamine and refluxing at 120° C. for 5 h, the reaction mixture was cooled to room temperature and stirred for 18 h, and the residual solvent was removed by distillation under reduced pressure. The final product obtained was purified by flash column chromatography (methanol / triethylamine=50:1 as eluent) to obtain cholic acid diamine.
[0066] (2) The preparation method of the clathrate is the same as step (3) of Example 2, wherein the main ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com