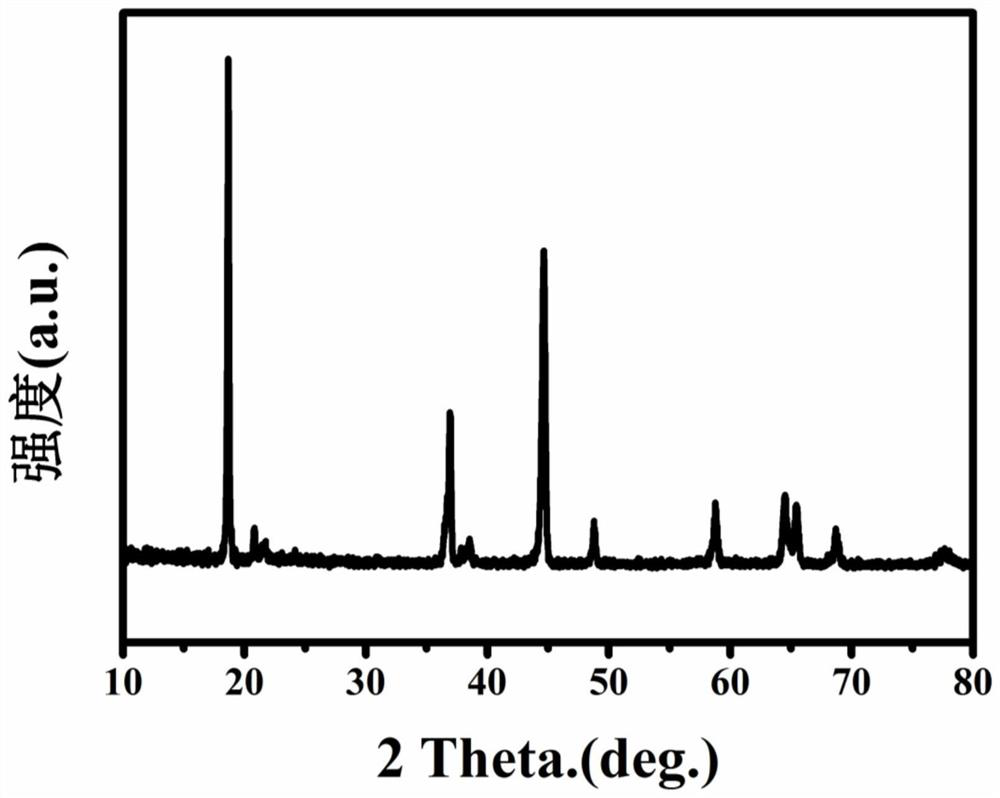
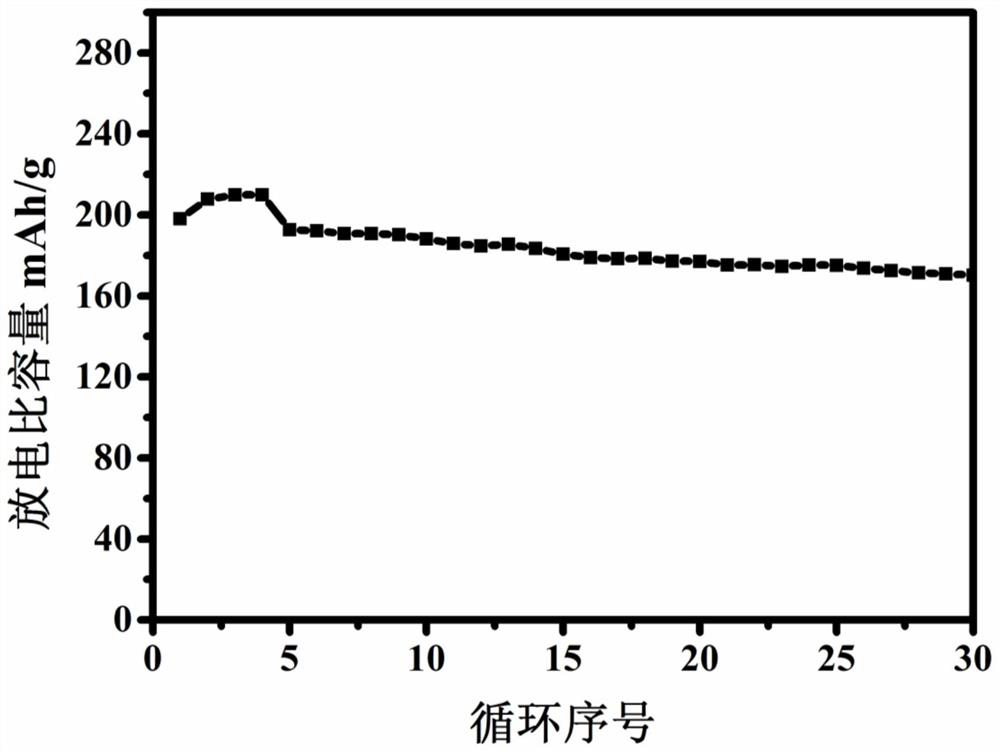
A method for regenerating lithium-rich manganese-based cathode materials using waste lithium batteries
A technology for waste lithium batteries and positive electrode materials, which is applied in the field of regenerating lithium-rich manganese-based positive electrode materials from waste lithium batteries, can solve the problems of complex preparation process and environmental pollution, and achieve the effects of high recycling efficiency, cost reduction, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This embodiment includes the following steps:
[0033] (1) Pretreat the waste nickel-cobalt-manganese ternary lithium-ion battery, including decomposing and disassembling the battery to obtain the positive electrode, and then peeling off the positive electrode material from the positive electrode, crushing and calcining to obtain the positive electrode material powder; 5g of the positive electrode material powder Mechanically mix 1g of graphite with a ball mill for 1 hour to obtain a powder uniformly mixed with graphite; sinter the powder uniformly mixed with graphite in an argon atmosphere at a sintering temperature of 800°C for 1 hour to obtain a powder containing lithium, nickel, cobalt and Mixed powder of manganese element;
[0034] (2) Mix 1 g of reduced powder with 18.375 g of NH 3 h 2 O, 7g NH 4 HCO 3 Add deionized water to 50ml, heat to 60°C in a water bath, leach for 8 hours under magnetic stirring, and filter to obtain the leachate. Take 1g of leaching re...
example 2
[0041] This embodiment includes the following steps:
[0042] (1) Pretreat the waste nickel-cobalt-manganese ternary lithium-ion battery, including decomposing and disassembling the battery to obtain the positive electrode, and then peeling, crushing and calcining the positive electrode material from the positive electrode to obtain the positive electrode material powder; mix 5g of the positive electrode material powder with 1g Graphite is mechanically mixed with a ball mill for 1 hour to obtain a uniformly mixed powder with graphite, and sintered in an argon atmosphere at 800°C for 1 hour to obtain a mixed powder containing lithium, nickel, cobalt and manganese;
[0043] (2) Take 1g of mixed powder and 18.375 g of NH 3 h 2 O, 7 gNH 4 HCO 3 , add deionized water to 50mL, heat to 60°C in a water bath, leaching for 8 hours under magnetic stirring, and filter to obtain leaching solution and manganese-containing leaching residue. Take 1g of leaching residue (according to IPC a...
example 3
[0048] This embodiment includes the following steps:
[0049] (1) Pretreat the waste nickel-cobalt-manganese ternary lithium-ion battery, including decomposing and disassembling to obtain the positive electrode, and then peel and crush the positive electrode material from the positive electrode to obtain the positive electrode material powder; use a ball mill to process 5g of positive electrode material powder and 1g of graphite Mix mechanically for 1 hour to obtain a uniform powder mixed with graphite, and sinter in an argon atmosphere at 800°C for 1 hour to obtain a mixed powder containing lithium, nickel, cobalt and manganese;
[0050] (2) Mix 1 g of mixed powder with 18.375 g of NH 3 h 2 O, 7 gNH 4 HCO 3 , add deionized water to 50mL, heat to 60°C in a water bath, leaching for 8 hours under magnetic stirring, and filter to obtain leaching solution and manganese-containing leaching residue. Take 1g of leaching residue (detected by IPC, the amount of manganese substance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com