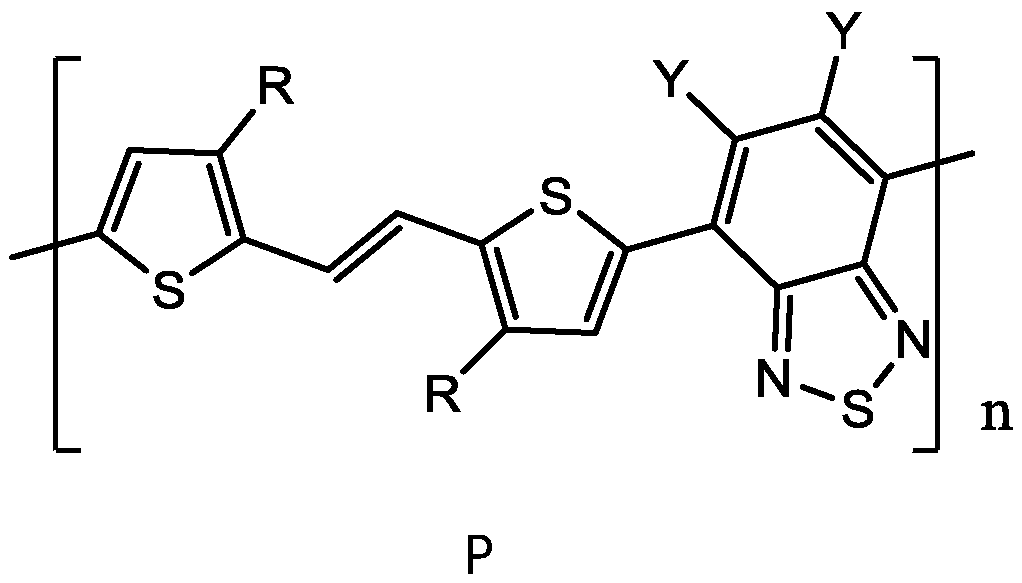
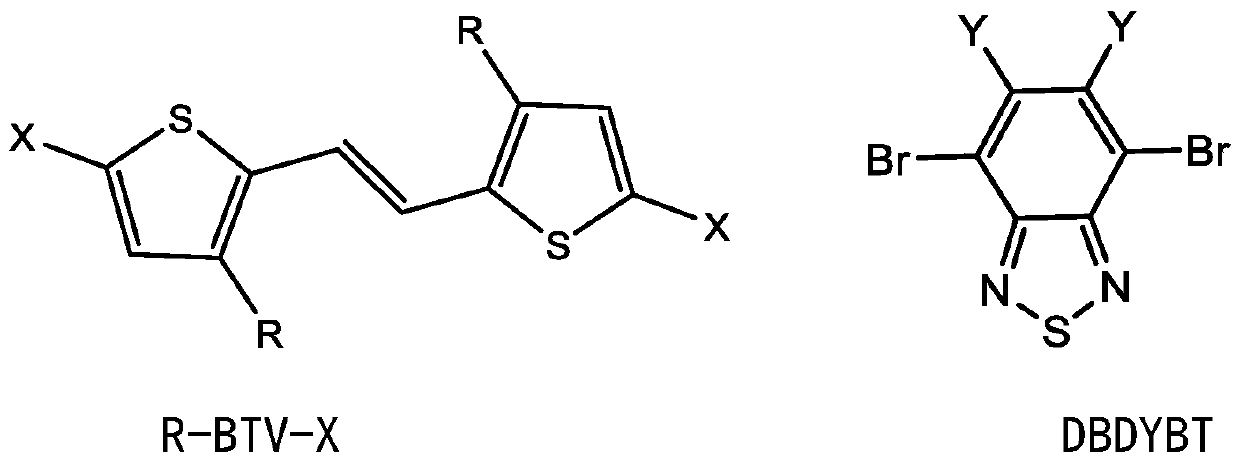
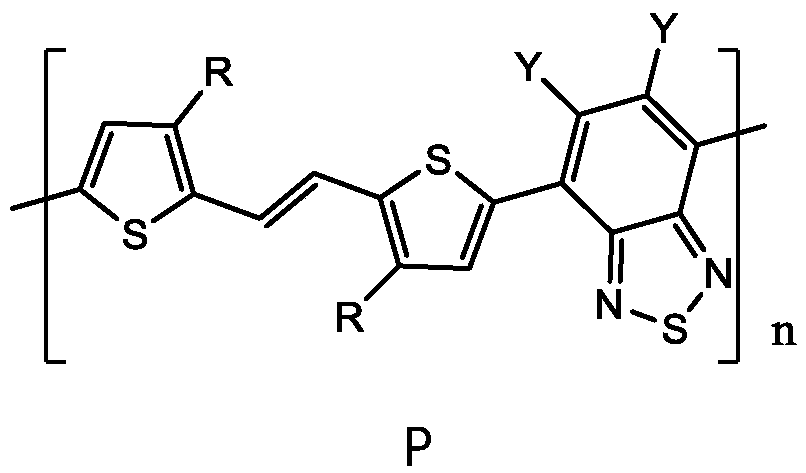
Polymer based on (E)-1, 2-di (2-thiophene) ethylene and benzothiadiazole as well as preparation method and application thereof
A technology for benzothiadiazole and polymers, which is applied in the field of polymers based on -1,2-diylethylene and benzothiadiazole and its preparation, can solve the problems of complex synthetic routes and raw materials, and achieve strong Promote and apply the value, enhance the conjugated structure, and improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0037] Example 1 Synthesis of polymer P1
[0038] A polymer based on (E)-1,2-bis(2-thiophene)ethylene and benzothiadiazole, specifically polymer P6 having the following general formula,
[0039]
[0040] The specific preparation process is as follows:
[0041] (1) Preparation C 12 H 25 -BTV-BO 2 C 6 H 12 : Under anhydrous and oxygen-free conditions, at a temperature of 0℃, add 3-bromothiophene (200mmol), dry THF (200ml) and LDA (240mmol) to the flask, react for 1h, then add dry DMF (240mmol) , React for 0.5h, after the reaction is over, cool to room temperature, overnight, after post-treatment, a brown liquid is obtained, which is the intermediate product 4-bromothiophene-2-carbaldehyde, and the yield is 45%.
[0042] Drop TiCl into the flask containing dry THF (20ml) 4 (20mmol), stir for 15min, then add Zn powder (35mmol), react at 65℃ for 1h, slowly add the intermediate product 4-bromothiophene-2-carboxaldehyde (12mmol) dropwise, and stir overnight. After post-treatment, a yellow po...
Example Embodiment
[0056] Example 2. Synthesis of polymer P2
[0057] A polymer based on (E)-1,2-bis(2-thiophene)ethylene and benzothiadiazole, specifically polymer P2 having the following general formula,
[0058]
[0059] The specific preparation process is as follows:
[0060] (1) Preparation C 8 H 17 -BTV-Sn(C 4 H 9 ) 3 : Under anhydrous and oxygen-free conditions, at a temperature of 0℃, add 3-bromothiophene (400mmol), dry THF (400ml), and LDA (480mmol) to the flask, react for 1h, then add dry DMF (480mmol) ), react for 0.5h, after the reaction is over, cool to room temperature, overnight, after post-treatment, a brown liquid is obtained, which is the intermediate product 4-bromothiophene-2-carbaldehyde, and the yield is 40%.
[0061] Drop TiCl into a flask containing dry THF (50ml) 4 (39.26mmol), stir for 15min, then add Zn powder (65.43mmol), react at 65℃ for 1h, slowly add the intermediate product 4-bromothiophene-2-carboxaldehyde (26.17mmol) dropwise, stir overnight, after post-treatment, a yel...
Example Embodiment
[0076] Example 3. Synthesis of polymer P3
[0077] A polymer based on (E)-1,2-bis(2-thiophene)ethylene and benzothiadiazole, specifically polymer P3 having the following general formula,
[0078]
[0079] The specific preparation process is as follows:
[0080] (1) Preparation C 10 H 21 -BTV-BO 2 C 6 H 12 : Under anhydrous and oxygen-free conditions, at a temperature of 0°C, add 3-bromothiophene (400mmol), dry THF (400ml) and LDA (480mmol) to the flask, react for 1h, then add dry DMF (480mmol) , React for 0.5h, after the reaction is over, cool to room temperature, overnight, after post-treatment, a brown liquid is obtained, which is the intermediate product 4-bromothiophene-2-carbaldehyde, and the yield is 40%.
[0081] Drop TiCl into the flask with dry THF (50ml) 4 (39.26mmol), stir for 15min, then add Zn powder (65.43mmol), react at 65℃ for 1h, slowly add the intermediate product 4-bromothiophene-2-carboxaldehyde (26.17mmol) dropwise, stir overnight, after post-treatment, a yellow c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com