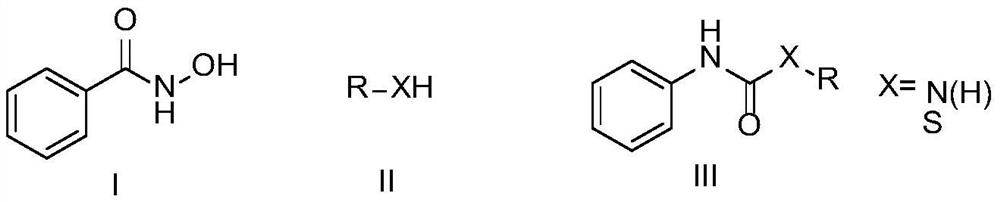
A kind of preparation method of amide derivative
A technology of amide derivatives and substances, applied in the field of asymmetric urea and thiocarbamate compounds, can solve the problems of unfavorable large-scale application, harsh reaction conditions, unfriendly environment, etc., and achieve a process suitable for large-scale preparation and operation Simple, good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
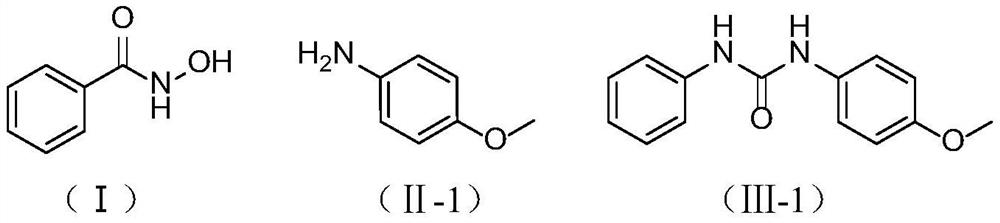
[0020] Embodiment 1: Preparation of 1-(4-methoxyphenyl)-3-phenylurea
[0021] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), p-methoxyaniline (II-1, X=N, R 2 = 4-OCH 3 -Ph) 17.24g (140mmol), 150mL water, 45.24g (2.5eq, 350mmol) DIPEA, in SO 2 f 2 In the atmosphere, stir at 25°C for 2h, filter after the reaction, and wash the filter cake with 10mL of acetonitrile until white to obtain 1-(4-methoxyphenyl)-3-benzene represented by formula (Ⅲ-1). Base urea 30.87g, yield 91%.
[0022] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.57(s,1H),8.46(s,1H),7.44(d,J=7.6Hz,2H),7.39–7.33(m,2H),7.27(t,J=7.9Hz ,2H),6.95(t,J=7.3Hz,1H),6.90–6.84(m,2H),3.36(s,3H).
[0023] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 154.93, 153.19, 140.36, 133.17, 129.20, 122.06, 120.49, 118.54, 114.45, 55.64.
[0024]
Embodiment 2
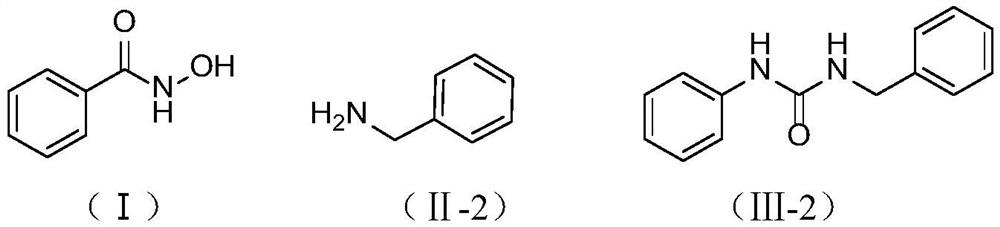
[0025] Embodiment 2: Preparation of 1-benzyl-3-phenylurea
[0026] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), benzylamine (II-2, X=N, R 2 =Ph-CH 2 -) 15.00g (140mmol), 150mL dichloromethane, 63.94g (3.0eq, 420mmol) DBU, in SO 2 f 2 In the atmosphere, stir at 25°C for 1 h. After the reaction, filter and rinse with 10 mL of acetonitrile until white to obtain (Ⅲ-2) 1-benzyl-3-phenylurea 26.g with a yield of 83%.
[0027] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.58(s,1H),7.44–7.40(m,2H),7.33(dt,J=10.9,7.1Hz,4H),7.27–7.18(m,3H),6.90(t, J=7.3Hz, 1H), 6.64(t, J=5.7Hz, 1H), 4.31(d, J=5.9Hz, 2H).
[0028] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 155.24, 140.46, 128.63, 128.29, 127.76, 127.10, 126.70, 121.07, 117.69, 42.73.
[0029]
Embodiment 3
[0030] Embodiment 3: Preparation of 3-phenyl-N-methyl-N-phenylurea
[0031] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), N-methylaniline (II-3, X=N, R 2=N-methylanilino) 15.00g (140mmol), 150mL acetonitrile, 18.10g (3.0eq, 420mmol) Na 2 CO 3 , at SO 2 f 2 In the atmosphere, stir at 30°C for 6h, filter after the reaction, rinse with 10mL of acetonitrile until white, and you can get 3-phenyl-N-methyl-N-phenylurea 25.98 shown in formula (III-3). g, yield 82%.
[0032] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.12(s,1H),7.46–7.38(m,4H),7.33(dd,J=8.4,1.1Hz,2H),7.27–7.20(m,3H),6.95(t, J=7.3Hz,1H),3.28(s,3H).
[0033] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 154.74, 144.09, 140.05, 129.24, 128.26, 126.22, 125.77, 122.04, 119.90, 37.55.
[0034]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap