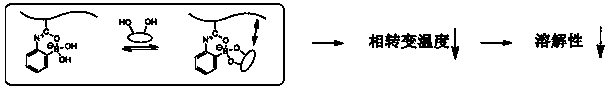
Glucose responsive polymer and preparation method thereof
A polymer and responsive technology, applied in the fields of polymer chemistry and material chemistry, can solve the problems of limiting the application of phenylboronic acid-containing glucose response systems, and achieve the effects of good glucose responsiveness, reduced solubility, and uniform chain length.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Wherein, the preparation method of the 2-acrylamidophenylboronic acid comprises: adding 2-aminophenylboronic acid and acryloyl chloride to the aqueous sodium hydroxide solution to adjust the pH of the solution to 6.5-7.5, and the obtained precipitate is the described 2-Acrylamidophenylboronic acid.
[0041] Wherein, the molar ratio of the 2-aminophenylboronic acid to acryloyl chloride is 1: (1-1.4).
[0042] The invention provides the glucose responsive polymer prepared by the preparation method.
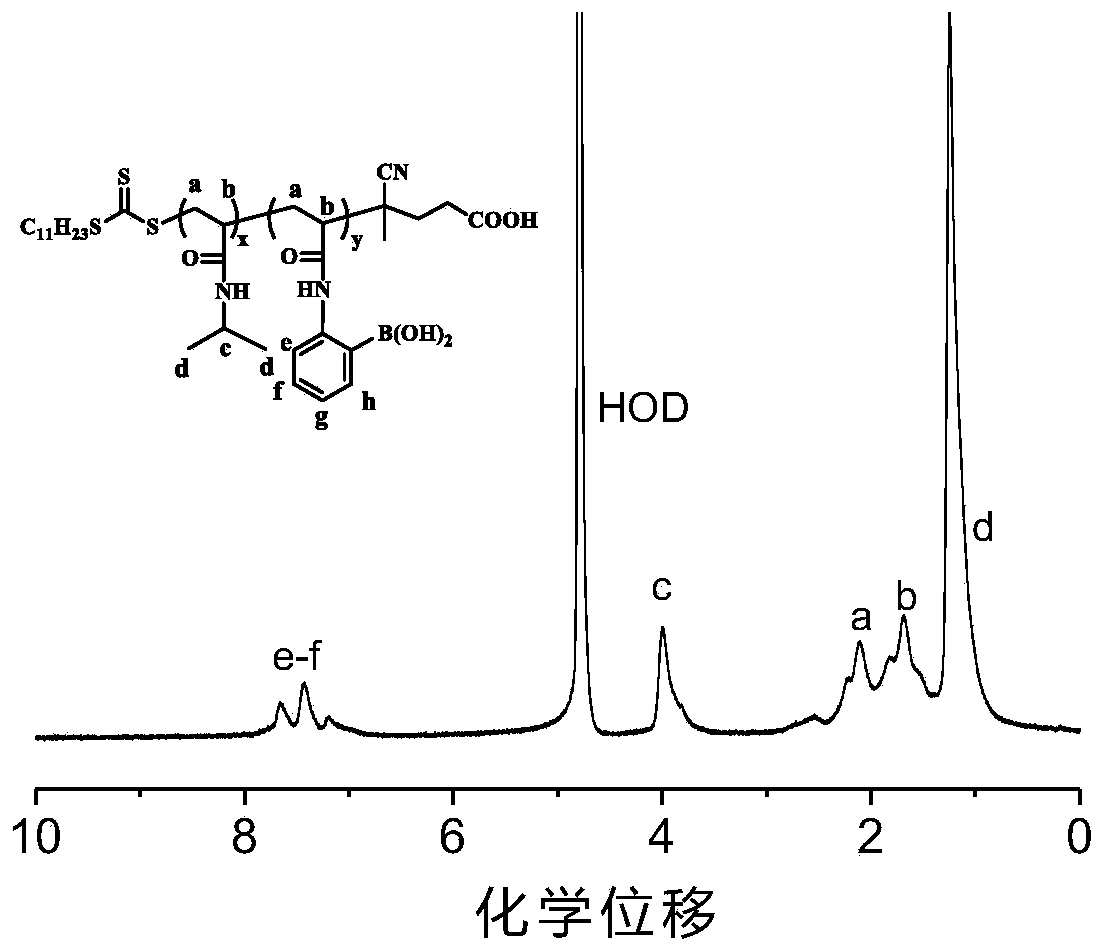
[0043] Wherein, the glucose-responsive polymer has the following general structural formula:
[0044]
[0045] Wherein, R is ethyl, n-hexyl, dodecyl; x is 85-380, y is 15-60.
[0046] Wherein, the number average molecular weight of the polymer is 12k-48k g / mol, and the dispersibility index of the polymer is <1.2.
[0047] In the following exemplary embodiments, the present invention provides a glucose responsive polymer and a preparation method thereof. The polymer is ...
Embodiment 1
[0062] In this example, N-isopropylacrylamide (NIPAM) and 2-acrylamidophenylboronic acid (2-AAPBA) were used to synthesize glucose-responsive polymer (P(NIPAM-co-2-AAPBA)) by RAFT polymerization. And demonstrate its responsiveness to glucose molecules, and describe the method in detail:
[0063] (A) Preparation of 2-acrylamidophenylboronic acid
[0064] Add 2-aminophenylboronic acid (1.08g, 6.2mmol) to 15mL aqueous sodium hydroxide solution (2M) and dissolve fully. After that, acryloyl chloride (1.0 mL, 12.6 mmol) was added dropwise in an ice-water bath. After reacting for 2 hours, the pH was adjusted to 6.5-7.5, and the 2-acrylamidophenylboronic acid product was precipitated. Suction filtration, washing and drying gave pure 2-acrylamidophenylboronic acid with a yield of ~50%.
[0065] (B) Synthesis of glucose responsive polymer (P(NIPAM-co-2-AAPBA))
[0066] Press N-isopropylacrylamide, 2-acrylamidophenylboronic acid, chain transfer reagent CDTPA, initiator AIBN molar rat...
Embodiment 2
[0070] (A) Preparation of 2-acrylamidophenylboronic acid
[0071] Add 2-aminophenylboronic acid (1.08g, 6.2mmol) to 15mL aqueous sodium hydroxide solution (2M) and dissolve fully. After that, acryloyl chloride (1.0 mL, 12.6 mmol) was added dropwise in an ice-water bath. After reacting for 2 hours, the pH was adjusted to 6.5-7.5, and the 2-acrylamidophenylboronic acid product was precipitated. Suction filtration, washing and drying gave pure 2-acrylamidophenylboronic acid with a yield of ~50%.
[0072] (B) Synthesis of glucose responsive polymer (P(NIPAM-co-2-AAPBA))
[0073] Press N-isopropylacrylamide, 2-acrylamidophenylboronic acid, chain transfer reagent CDTPA, join in the Schlenk tube in the ratio of initiator AIBN molar ratio 360:40:1:0.2, add methanol / water solvent (95: 5 volume ratio) dissolved. Freezing and thawing pump circulation method was used to degas three times, and reacted at 70°C for 24 hours. The reaction was terminated by cooling down in an ice-water ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com