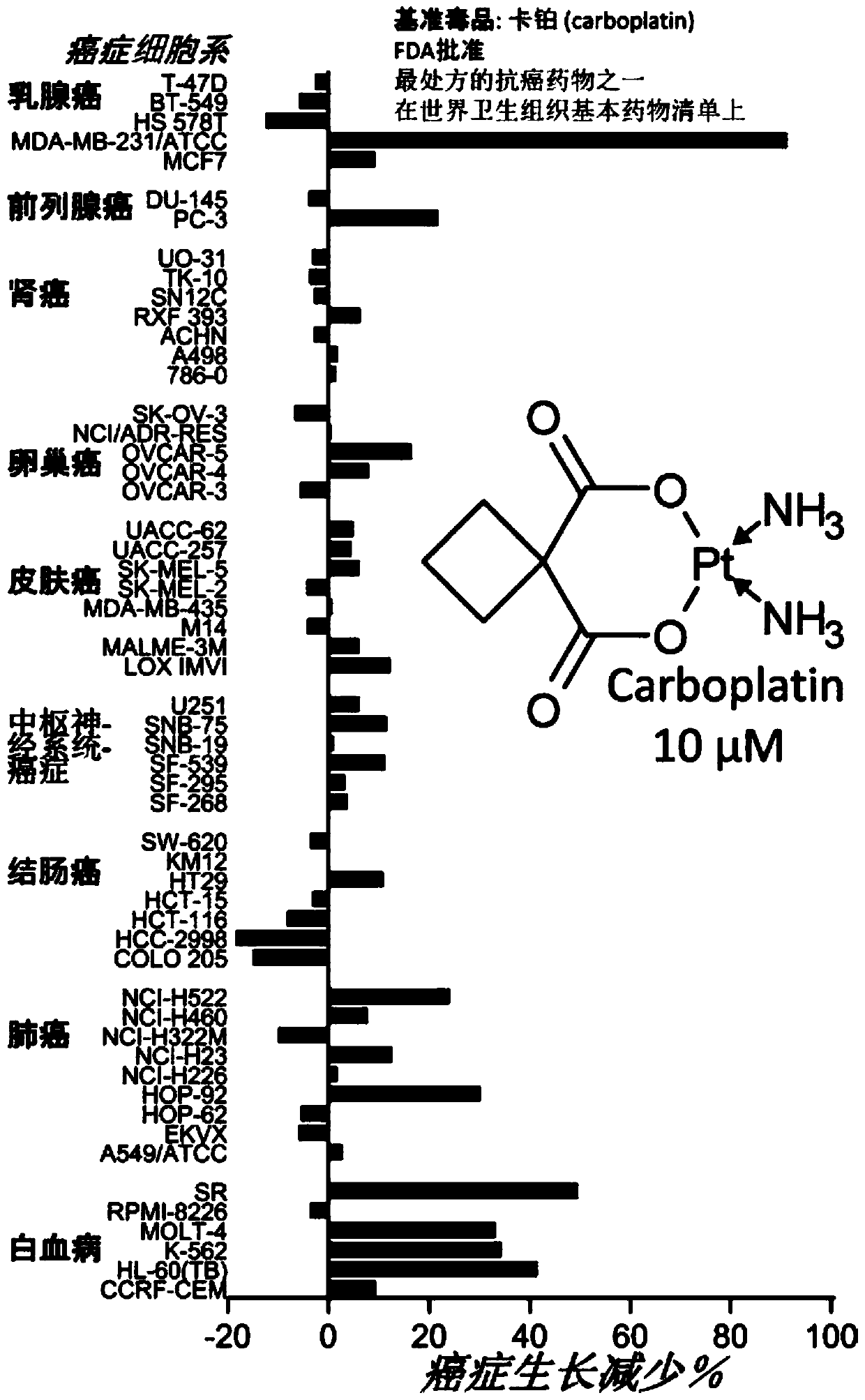
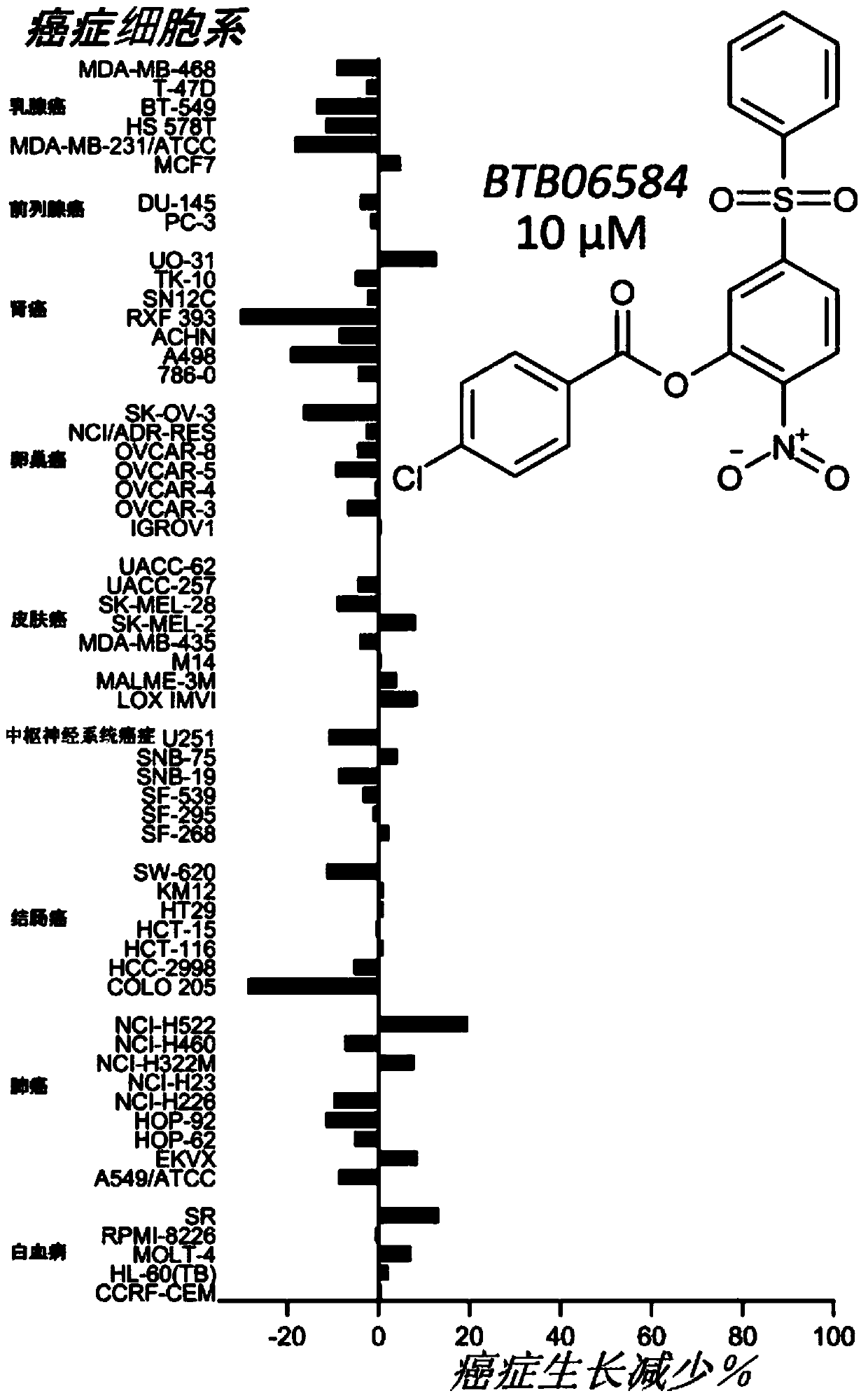
Therapeutic modulators of the reverse mode of atp synthase
A compound and composition technology, applied in the direction of drug combination, organic active ingredient, heterocyclic compound active ingredient, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0186]
[0187] The structure on the left has a low EC50 against F1F0 ATP hydrolysis (0.018 μΜ), with a [EC50F1F0 ATP synthesis / EC50F1F0 ATP hydrolysis] ratio >5556. In rats, the drug (administration polyethylene glycol:water:ethanol, 1:1:1) was orally bioavailable (47%) with good pharmacokinetics (blood = 2.1 hours after intravenous administration of the drug half-life, Cmax is 21 microns, volume distribution = 2.39 liters / kg). On the right, where the hydrogen atom on the chiral carbon is replaced by deuterium, the deuterated analogue (KIE, [37]) that confers greater stereoisomer stability because of kinetic isotope effects is more preferred. Greater % deuterium enrichment at the chiral carbon (21 carbon atoms) and greater enantiomeric excess, more preferred embodiments. The other atom or isotope in other preferred embodiments positions the hydrogen at the chiral carbon, blocking its racemization, ensuring a permanent stereomeric excess. For example, fluorine. or carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com