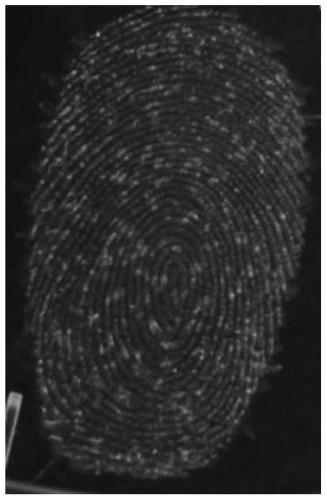
Application of diarylethene compound in fingerprint fluorescence color development
A diarylethene compound technology, applied in the application field of diarylene compounds in fingerprint fluorescent color development, to achieve the effect of increased polarity, high contrast, and not easy to aggregate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] A compound is compound T1, and the representative method of synthesizing compound T1 is as follows:
[0168]
[0169] Compound 1 (200 mg, 0.733 mmol, 1 eq.), compound 2 (159 mg, 0.733 mmol, 1 eq.) and piperidine (2 drops) in EtOH (3 mL) were reacted at 80 °C for 16 h. It was concentrated under reduced pressure, and the crude product was purified by flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0-15%) to obtain red solid compound T1 (220 mg, yield 64%). Mass spectrum (ESI, m / z): 393.3 (M-Br) + .
[0170] Compound T1 is hydrophilic, soluble in water, and has a certain oil solubility, for example, it can be well dissolved in methanol, ethanol, DMF, DMSO, acetonitrile, tetrahydrofuran, dichloromethane and other oil-soluble solvents; insoluble in n-hexane , ether and ethyl acetate and other less polar solvents. Compound T1 has almost no fluorescence in aqueous solution, and emits strong orange-red fluorescence in 99% ethyl acetate / water syste...
Embodiment 2
[0180] A diarylethene compound T2, which has the structural formula shown below:
[0181]
[0182] Methods of synthesizing this compound:
[0183] Compound 3 (363 mg, 1.00 mmol, 1 eq.), compound 4 (262 mg, 1.00 mmol, 1 eq.) and piperidine (3 drops) in EtOH (5 mL) were reacted at 80 °C for 16 h. Concentrated under reduced pressure, the crude product was purified by flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0-18%) to give a red solid (271 mg, yield 48%). Mass spectrum (ESI, m / z): 483.3 (M-Br) + .
[0184] The compound is amphiphilic, with weak fluorescence in aqueous solution and strong orange-red fluorescence in 99% ethyl acetate / water system, and has a remarkable aggregation-induced luminescence effect.
[0185] The compound fluoresces strongly for the word lipids, indicating that this probe can indeed identify lipids in sweat latent fingerprints.
Embodiment 3
[0187] A diarylethene compound T3, it has the structural formula shown below:
[0188]
[0189] Methods of synthesizing this compound:
[0190] Compound 5 (546 mg, 2.00 mmol, 1 eq.), compound 6 (432 mg, 2.4 mmol, 1 eq.) and piperidine (6 drops) in MeCN (30 mL) were reacted at 80 °C for 16 h. Concentrated under reduced pressure, the crude product was purified by a flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0-20%), and the isolated intermediate product T3-1 was dissolved in 50 mL of a mixed solution of ethanol / water (volume ratio: 9:1), add LiOHH 2 O (419 mg, 10 mmol), after stirring at room temperature overnight, 1M hydrochloric acid was added dropwise to adjust the Ph value to 2-3. The organic solvent was removed by rotary evaporation, extracted three times with dichloromethane, and the combined extracts were concentrated and separated by fast liquid preparative chromatography (mobile phase gradient: DCM / MeOH=0~25%) to obtain a red solid (254...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com