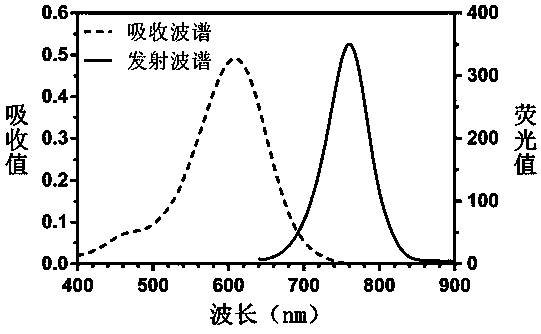
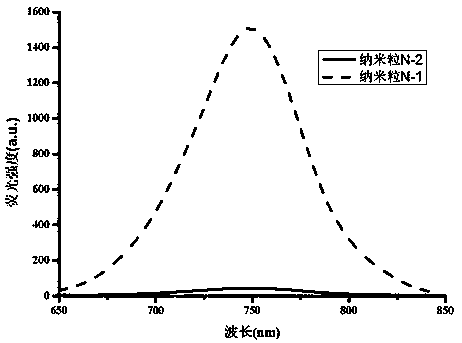
Patents
Literature
87results about How to "Reduce background fluorescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Heptamethine cyanine active fluorescent probe and preparation method and application thereof
InactiveCN108033907AImprove stabilityReduce background fluorescenceOrganic chemistryFluorescence/phosphorescenceChemical reactionStructural formula
The invention relates to a heptamethine cyanine active fluorescent probe and a preparation method and application thereof. The structural formula of the heptamethine cyanine active fluorescent probe is as shown in the specification, wherein X=II-IX; each of R1 and R2 is (CH2)mCH3, (CH2)nOH, (CH2CH2O)pCH3 and CH2C6H5; each of R3 and R4 is H, SO3H, SO3Na and SO3K; each of a, b, c, d, e, f and g is 2-8; each of n, m and p is 1-10. The heptamethine cyanine active fluorescent probe has the advantages that the heptamethine cyanine active fluorescent probe is based on near-infrared long-wave heptamethine cyanine dye, indoline is selected as the aroma parent nucleus to increase fluorescence intensity, and methenyl chain intermediate cyclohexene rigid bridging enhances stability; nitrogen derivatives with chemical reactivity sites are used to perform nucleophilic substitution on the meso-position of the heptamethine cyanine parent dye, and accordingly Stokes shift and active chemical groups areincreased greatly to facilitate the fluorescent labeling of various substances; the fluorescent probe is of a symmetrical structure, preparation and purification processes are simplified, and cost islowered favorably; the probe can be used as the fluorescent labeling probe of biological molecules such as high-sensitivity protein, sugar and DNA and nano carriers to perform cell or living-body horizontal fluorescence imaging, and the like.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
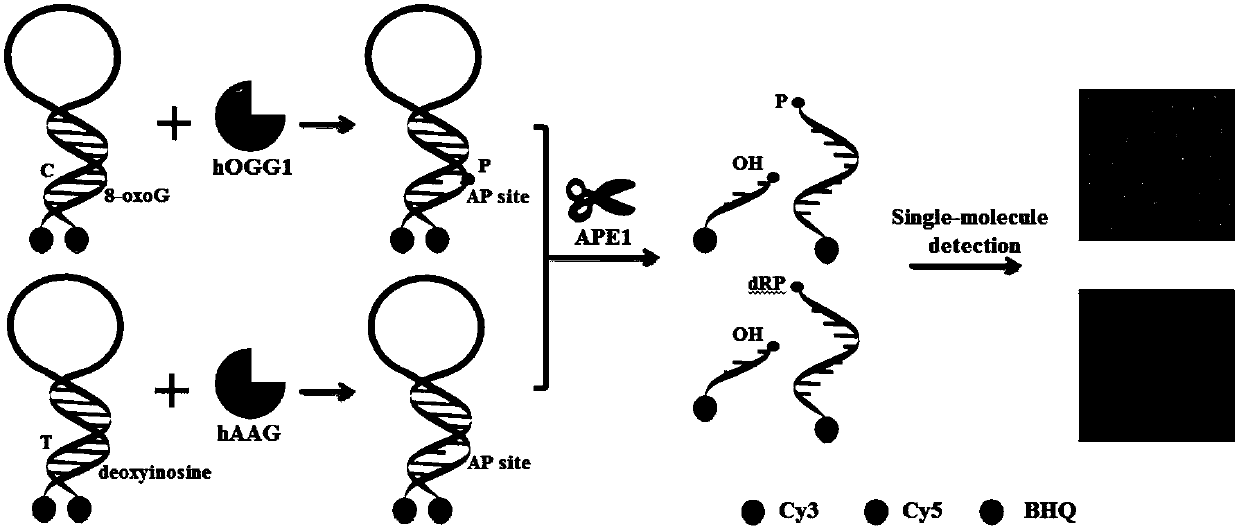
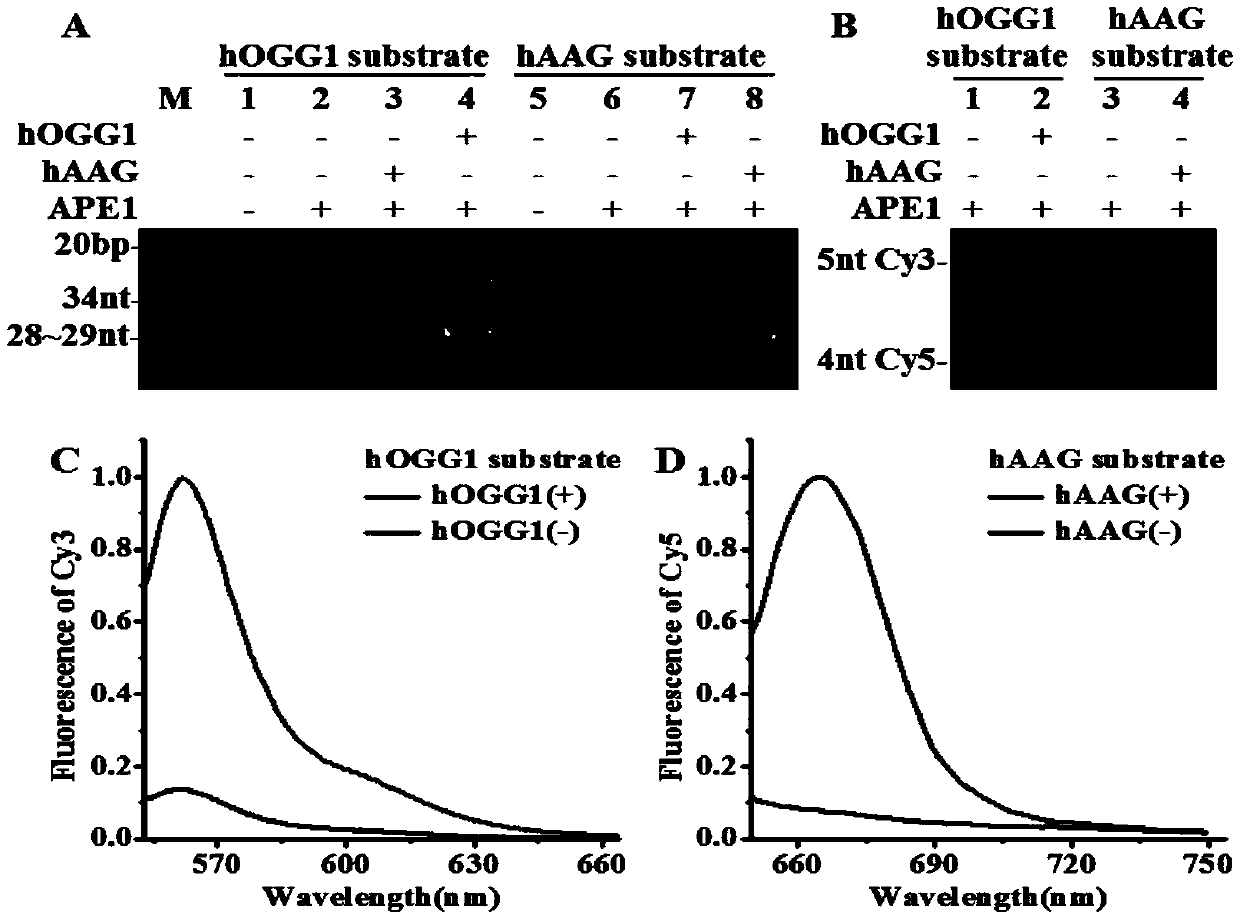
Fluorescence chemical sensor and method for simultaneously detecting diversified DNA (deoxyribonucleic acid) glycosylases on single-molecular levels and application of fluorescence chemical sensor
ActiveCN107723338AEasy to prepareThe detection method is simpleMicrobiological testing/measurementFluorescence/phosphorescenceMethylpurine DNA GlycosylaseMolecular level
The invention discloses a fluorescence chemical sensor and a method for simultaneously detecting diversified DNA (deoxyribonucleic acid) glycosylases on single-molecular levels and application of thefluorescence chemical sensor. The fluorescence chemical sensor, the method and the application have the advantages that the fluorescence chemical sensor is based on two different molecular beacons including a molecular beacon modified by 8-hydroxyl guanine and a molecular beacon modified by deoxygenated hypoxanthine, the tail end of the molecular beacon modified by the 8-hydroxyl guanine is labeled by cyanine 3 (Cy3) and quenching groups, the tail end of the molecular beacon modified by the deoxygenated hypoxanthine is labeled by cyanine 5 (Cy5) and quenching groups, the fluorescence chemicalsensor is used for detecting 8-hydroxyl guanine DNA glycosylases and N-methylpurine DNA glycosylases and is different from the traditional molecular beacons which can be severely affected by dynamicsand thermodynamics, signal restoration of the Cy3 and the Cy5 depends on molecular beacon splitting with the DNA glycosylases used as media, the DNA glycosylases can be simultaneously sensitively detected by the aid of the method without optional signal amplification, the activity of hOGG1 and hAAG can be detected by the aid of the method in an ultra-sensitive manner without optional signal amplification, the fluorescence chemical sensor can be easily, conveniently and quickly operated, and accurate and reliable test results can be obtained.
Owner:SHANDONG NORMAL UNIV
Protein microarray slide
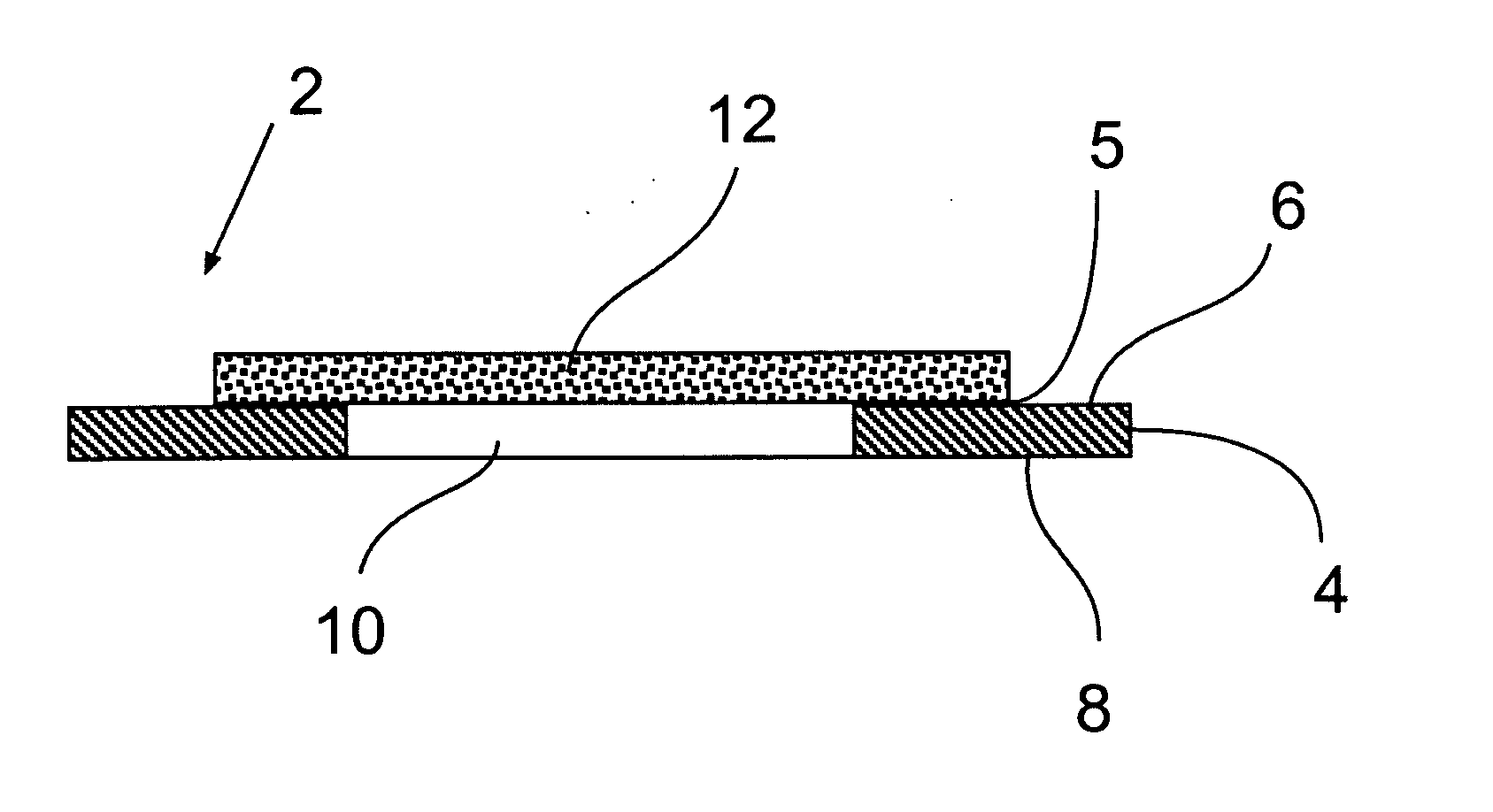
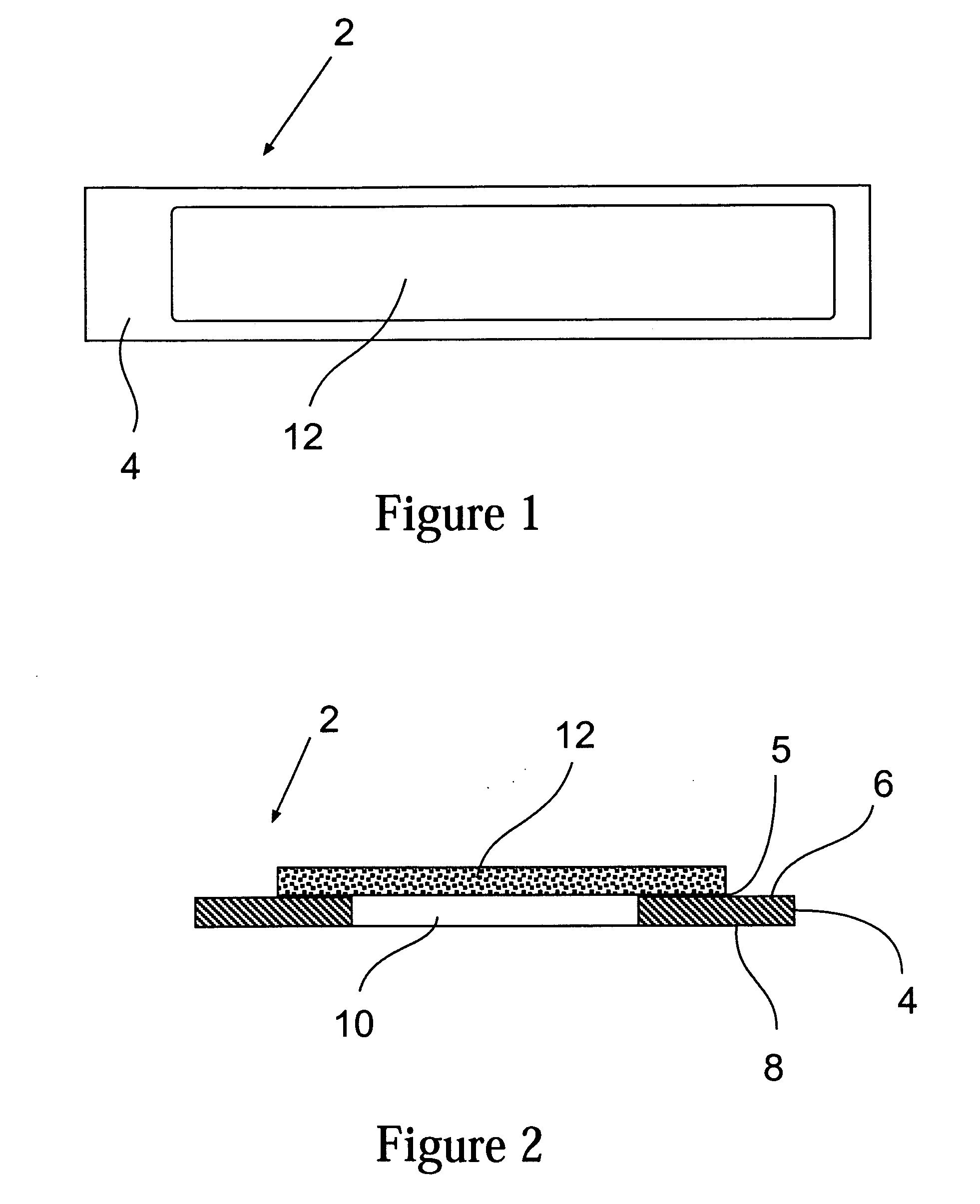
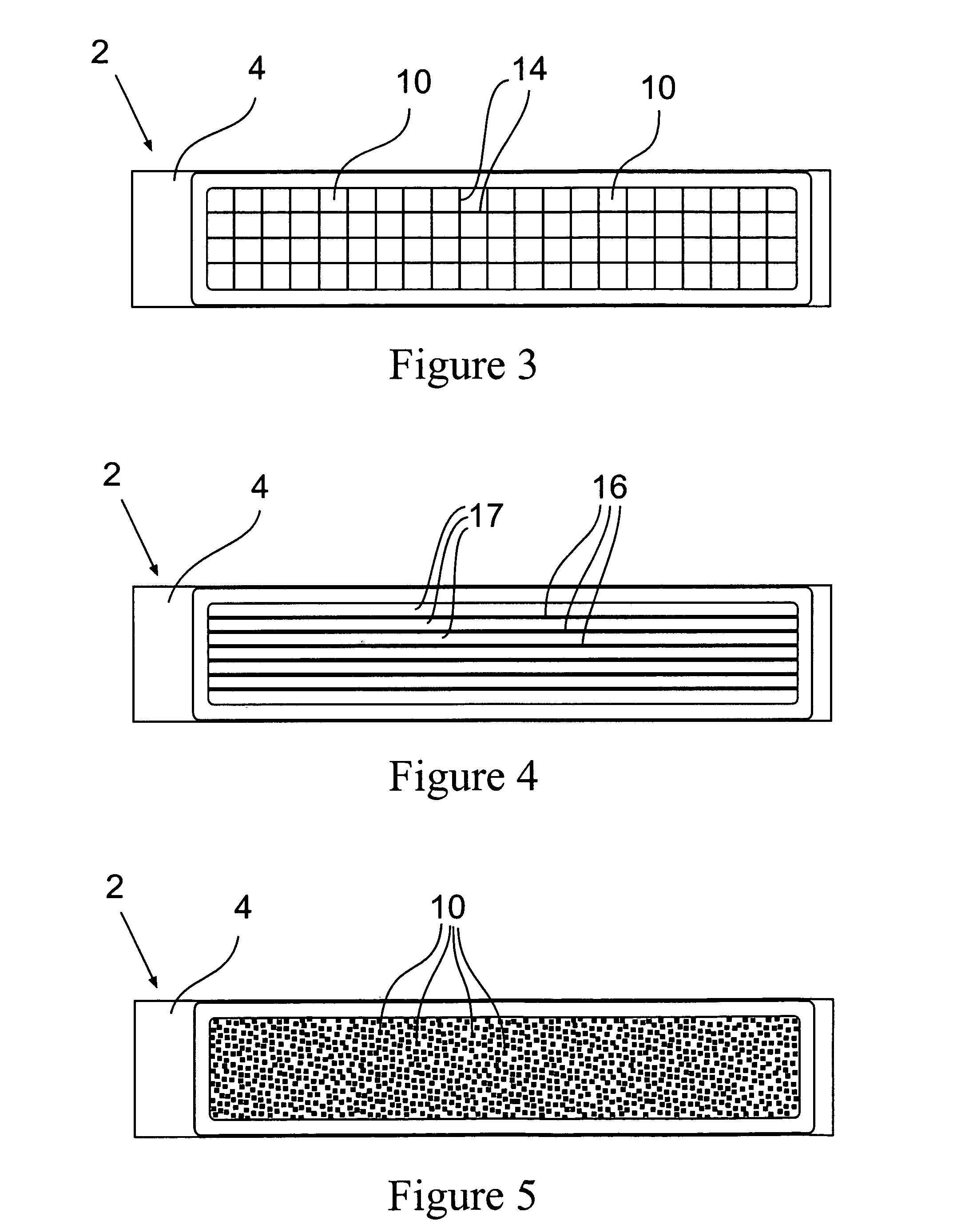
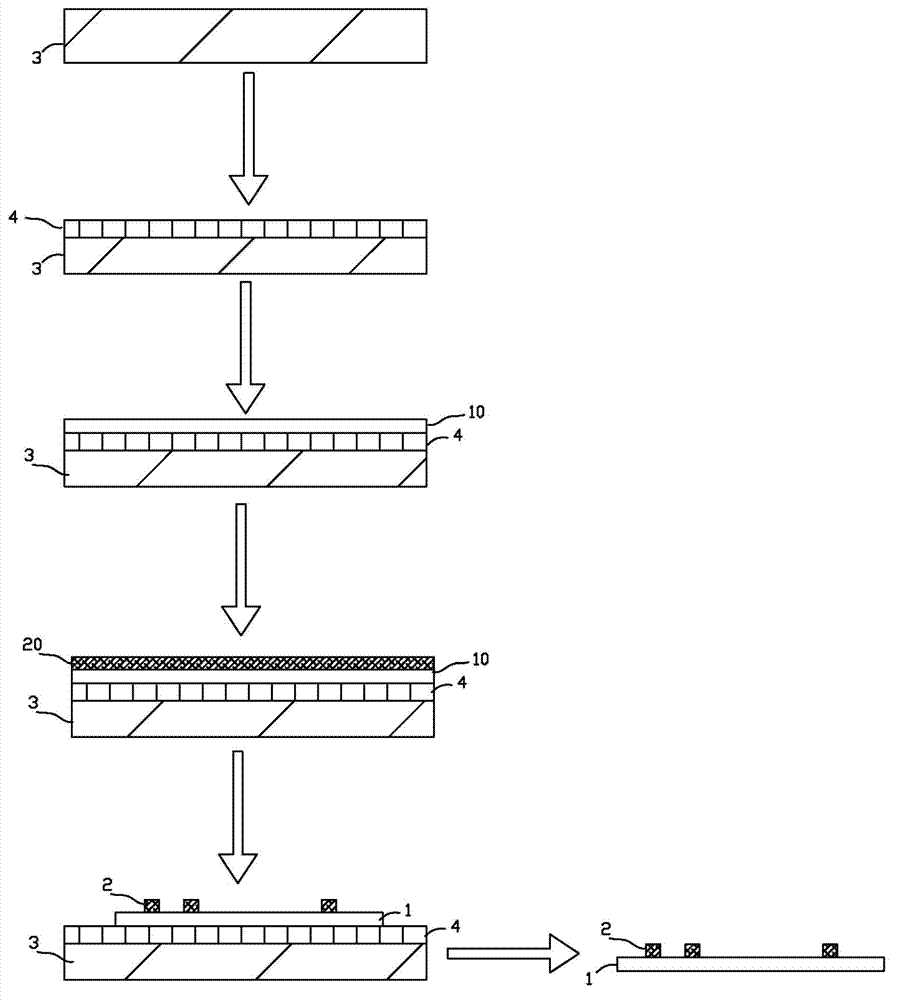
InactiveUS20070128069A1Reduce the amount requiredImproves background-fluorescence performanceSequential/parallel process reactionsAnalysis using chemical indicatorsAdhesiveEngineering
The present invention relates to a slide having a first and a second major surface on opposite sides of the slide and one or more openings through the slide from the first major surface to the second major surface and one or more membranes mounted to one major surface of the slide over the one or more openings. The slide provides for both an ability to wash and to reduce the background fluorescence in the active membrane area. Preferably the membrane is made of nitrocellulose, nylon or PVDF. The opening(s) can be used to allow wash solutions to pass through the membrane and be removed along with the contaminants (unattached materials such as proteins or antibodies, dyes, salts, etc) from the slide thereby reducing the fluorescent background. Additionally, the one or more openings exhibit a lower fluorescence than a membrane that is laid over or attached to the slide, as neither the slide material or any adhesive is present which would otherwise increase the background fluorescence.
Owner:MILLIPORE CORP
Fungus detection fluorescent dyeing liquid and use thereof
InactiveCN106198470AClear structureEasy to findPreparing sample for investigationFluorescence/phosphorescenceParaffin waxFluorescence
The invention discloses a fungus detection fluorescent dyeing liquid and a use thereof. The fungus detection fluorescent dyeing liquid comprises an independent dyeing liquid A and an independent re-dyeing liquid B. The dyeing liquid A comprises, by weight, 0.02-0.043% of a fluorescent brightener, 1-10% of potassium hydroxide, 10-20% of dimethyl sulfoxide, 0-5% of glycerin and 65-87% of water. The re-dyeing liquid B comprises, by weight, 0.1-0.5% of Evans blue and 99.50-99.90% of water. The invention discloses a use of the fungus detection fluorescent dyeing liquid. The invention provides a novel fast fungus infection detection product. The product can be used for detecting various fungi which may exist in a fresh or frozen clinical sample and paraffin and ethylene glycol methacrylate-coated tissue.
Owner:JIANGSU LIFETIME BIOLOGICAL TECH
Method, oligonucleotide and kit for detecting high-risk HPV (human papilloma viruses)
ActiveCN105603121AReduce usageGuaranteed SensitivityMicrobiological testing/measurementMicroorganism based processesTrue positive ratePhylogenetic tree
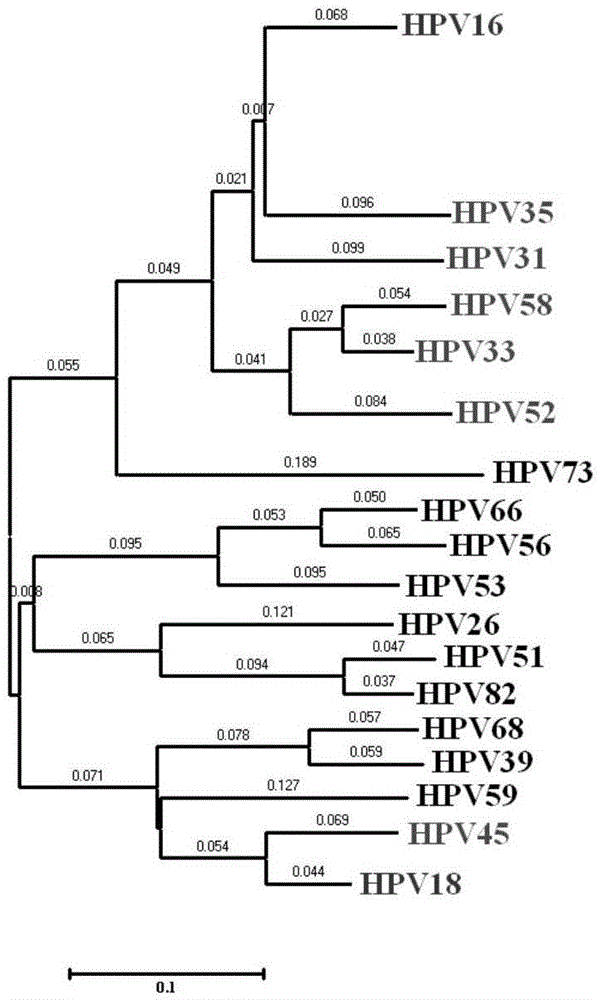
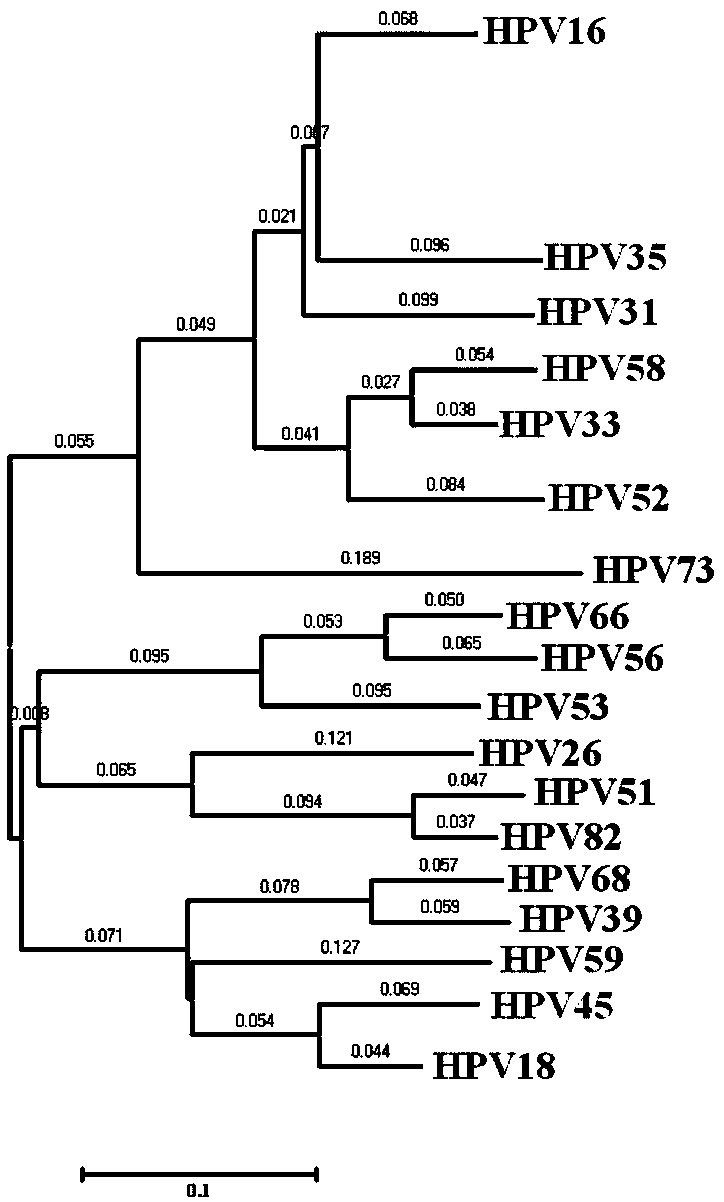
The invention provides a method, oligonucleotide and kit for detecting common high-risk HPV (human papilloma viruses). A fluorescence PCR (polymerase chain reaction) technology is adopted, HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 26, 53, 66, 73 and 82 which possibly exist in a sample are subjected to initial detection and type identification. By the method, oligonucleotide and kit for detecting the common high-risk HPV, 18 high-risk types can be detected simultaneously, and the HPV 16 and 18 can be subjected to genetic typing simultaneously. On the basis of 18 types of high-risk HPV and affinity of other types on a phylogenetic tree, a high-risk HPV primer and a probe are designed, a background fluorescence value is reduced remarkably while detection accuracy, sensitivity and specificity are guaranteed, detection flux is increased remarkably, and detection cost is greatly reduced.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Method for producing fluorescent/colorimetric dual-pattern paper chips for detection of cancer cell and cell surface polysaccharide
InactiveCN106092982AReduce background fluorescenceLarge specific surface areaMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceAptamerWax
The invention discloses a method for producing fluorescent / colorimetric dual-pattern paper chips for detection of cancer cell and cell surface polysaccharide. According to the method, the paper chips are produced by a wax printing technology, gold nanorods and porous silver and aptamers are grown in a fluorescent layer work area, then cancer cell capture is carried out, a multi-branched hybridization technology is used for amplification of signal molecule, and accurate measurement of the cancer cells can be realized, the produced paper chips are folded, a chromogenic solution is dropped in a reaction area, and thereby visualization pre-measurement of the cancer cells can be realized. A polysaccharide inhibitor is added, and dynamic symptom of cell surface polysaccharide is realized.
Owner:UNIV OF JINAN
Adenosine determination method based on fluorescent and colorimetric dual detection system
ActiveCN104155273AReduce background fluorescenceGood quenching effectMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceAdenosineSulfur
The invention discloses an adenosine determination method based on a fluorescent and colorimetric dual detection system, and belongs to the technical field of analytical chemistry. By gold-sulfur bonds, sulfydryl DNAs are connected to gold nanoparticles, and the sulfydryl DNAs can hybridize with adenosine aptamers modified by fluorescent dye; because of fluorescence quenching in high-salt solution, the detection system is free of fluorescence or very low in fluorescence intensity and the stable gold nanoparticles keep the solution in a wine red color; addition of adenosine causes changes in the structures of the aptamers, so that the adenosine aptamers modified by the fluorescent dye are dissociated from the gold nanoparticles and the fluorescence of the fluorescent dye recovers; at the moment, the gold nanoparticles cannot exist stably, the color of the solution changes from red to blue. By the adenosine determination method, through changes in fluorescence intensity and solution color of the detection system, qualitative and quantitative adenosine determination is achieved; the adenosine determination method has the advantages of high sensitivity, good selectivity and the like.
Owner:UNIV OF SCI & TECH BEIJING
Coding suspension microchip and preparation method and application thereof
ActiveCN102788779AEasy to fixReduce background fluorescenceChemiluminescene/bioluminescenceFluorescencePhysical chemistry
The invention discloses a coding suspension microchip and a preparation method and application thereof. The microchip comprises a transparent substrate and a non-transparent plane microstructure serving as graph identification codes, and the non-transparent plane microstructure is distributed on the surface of the transparent substrate. Preferentially, the non-transparent plane microstructure comprises a high light reflection stacking structural layer formed on the surface of the transparent substrate. The microchip can be manufactured through a semiconductor micromachining process, an application mode of the microchip is that probe molecules suspended on the microchip in a liquid phase system are used for capturing molecules of objects to be measured, then the microchip is separated from the liquid phase system and washed, and finally images of the microchip are formed in a visible light channel and a fluorescence channel respectively to determine identification of target molecules captured by the microchip and conduct quantitative analysis on the target molecules. Inexpensive manufacture of the coding suspension microchip with high throughput and good detection accuracy is achieved, and the coding suspension microchip can be applied to simultaneous detection of multiple biology / medical indexes in liquid samples.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
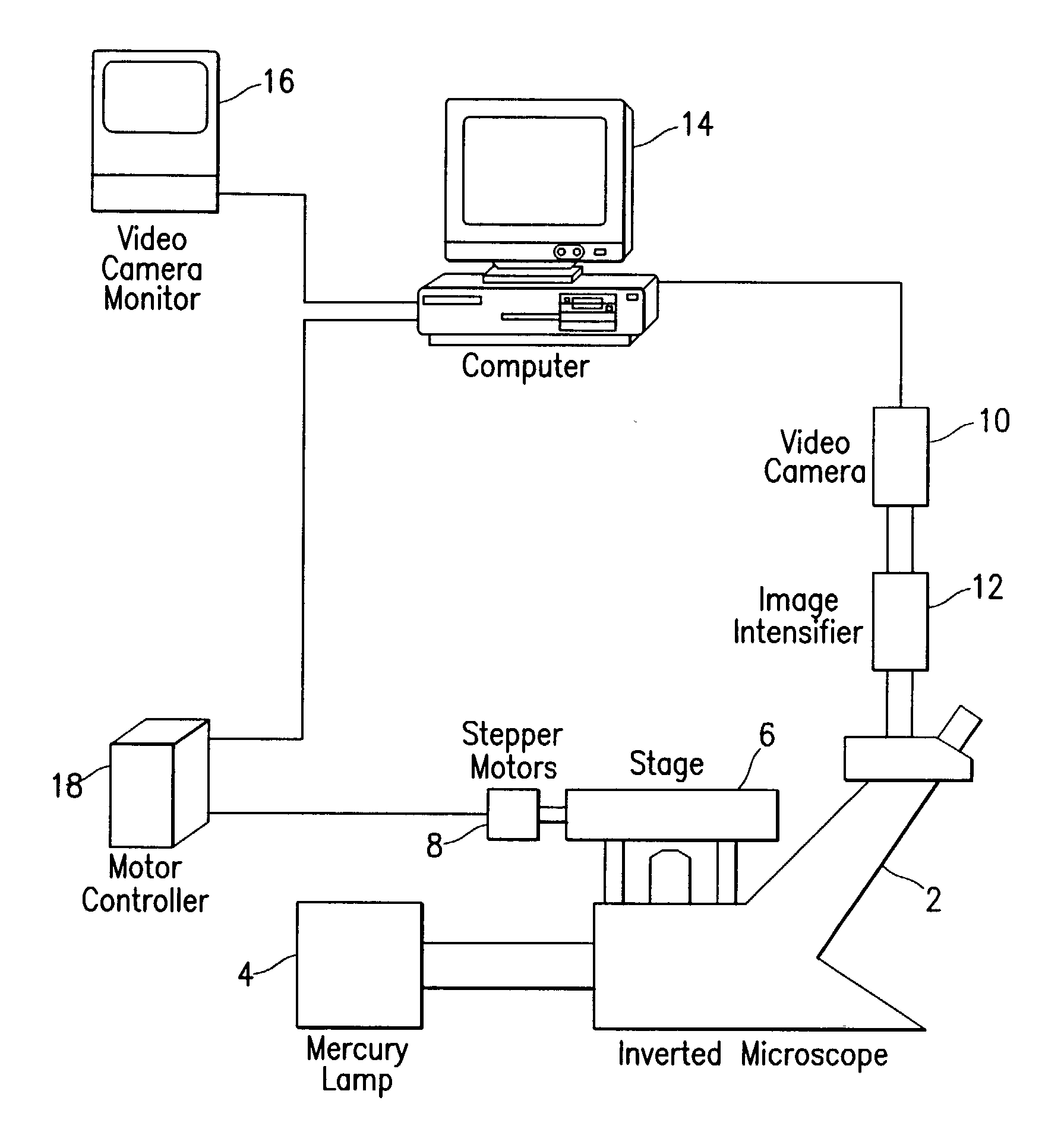
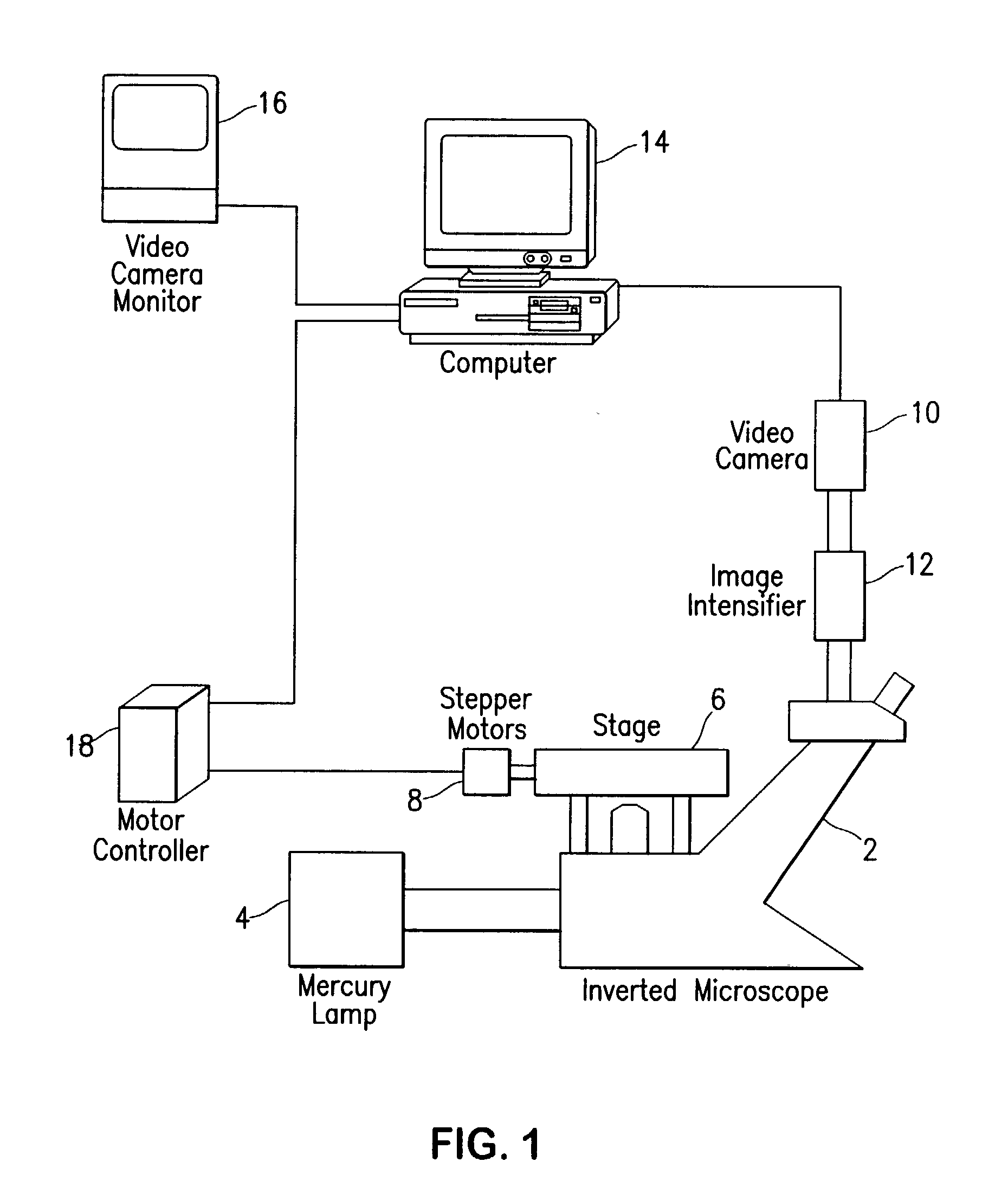
Fluorescence digital imaging microscopy system
InactiveUS20020191824A1High degreeIncrease rangeMicrobiological testing/measurementPreparing sample for investigationDigital imagingEosin
A method of preparing cell samples for viable cell number quantification with a fluorescence digital imaging microscopy system employing digital thresholding technique. The cell sample is stained with a first, fluorescent dye and treated with a second dye that is able to quench the fluorescence of the first dye. The fluorescent dye accumulates in viable cells only and is used to stain the viable cells. The second dye is excluded from viable cells but enters non-viable cells, thereby quenching the background fluorescence in non-viable cells and the medium. Two examples of dye combinations are described: fluorescein diacetate used as the fluorescent dye with eosin Y as the quenching dye; and calcein-AM used as the fluorescent dye with trypan blue as the quenching dye. By reducing the background fluorescence, the dynamic range and accuracy of viable cell number measurements are enhanced. In low viability cultures treated with fluorescein diacetate, background fluorescence completely masked viable cells, but digital thresholding and eosin treatment dramatically reduced background fluorescence, producing a linear response over 4 logs of viable cell density.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES
AIE composite electrostatic spinning fibrous membrane and preparation method and application thereof
ActiveCN110787316AFluorescence enhancementGood biocompatibilityPharmaceutical delivery mechanismElectro-spinningPolymer scienceAntibacterial activity
The invention provides an AIE composite electrostatic spinning fibrous membrane and a preparation method and application thereof. The AIE composite electrostatic spinning fibrous membrane is a nanofiber membrane formed from a biocompatibility polymer and an AIE molecule, can slowly release antibacterial activity component AIE molecules in the using process, can effectively restrain and kill bacteria, is free from any side effect on generation of normal cells, can promote adhesion, growth and proliferation of the cells, has favorable bacteria resistance and air permeability, can be completely jointed to the surface of wounds to maintain stable physiological environment, and can increase the healing speed. The AIE composite electrostatic spinning fibrous membrane is prepared by an electrostatic spinning technique, is simple in preparation method, low in cost, and suitable for large-scale industrial production, and has important application value in the respects for treating chronic wounds of wounds or burns and the like.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Construction of ratio-type three-dimensional metal-enhanced fluorescence Pb<2+> biosensor
The invention discloses preparation of a ratio-type three-dimensional metal-enhanced fluorescence Pb<2+> paper chip and application of equipment thereof in Pb<2+> detection. According to the preparation, a hydrophilic working area and a hydrophobic area of a paper chip are prepared by a wax printing technology, and further decahedral silver grows in the working area, the fluorescence intensities of two fluorescence signals are determined by means of layer-by-layer modification, the Pb<2+> is detected portably, reliably and sensitively by the relation curve of variation of two fluorescence intensity ratio over Pb<2+> concentration.
Owner:UNIV OF JINAN
Human intestinal tract carboxylesterase activity detection fluorescent probe substrate and use thereof
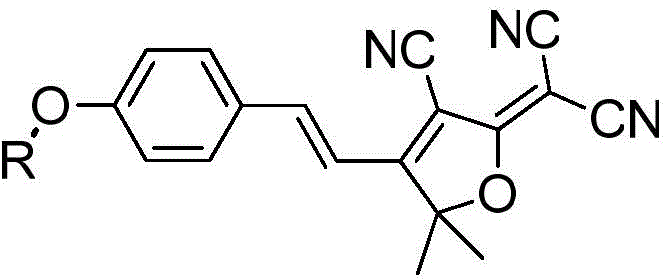
ActiveCN105441062AReduce background fluorescenceHigh sensitivityOrganic chemistryMicrobiological testing/measurementMetaboliteQuantitative determination
The invention relates to a human intestinal tract carboxylesterase activity detection fluorescent probe substrate and a use thereof and belongs to the technical field of medicine. The specific probe substrate is an aromatic ester (TCFE) of (E)-2-(4-(4-hydroxystyryl)-3-cyano-5, 5-dimethylfuran-2(5H)-ylidene)malononitrile (TCF). The substrate can be used for fast quantitative detection of human intestinal tract carboxylesterase activity and fast screening and assessment of a human intestinal tract carboxylesterase inhibitor. The fast quantitative determination process of human intestinal tract carboxylesterase activity comprises selecting a TCFE hydrolysis reaction as a probe reaction, adding an appropriate substrate into a buffer system containing hCE2, and determining real activity of hCE2 enzyme in various external biological samples through quantitative determination of a hydrolysis metabolite TCF generation amount in unit time in a linear reaction zone. The probe has good selectivity and the hydrolysate TCF has long fluorescence emission wavelength of 612nm. The human intestinal tract carboxylesterase activity detection fluorescent probe substrate can reduce biological sample background fluorescence and improve sensitivity and has good practicality and application prospect.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI +1
Anionic type iridium complex for oxygen sensing, and preparation method and application thereof
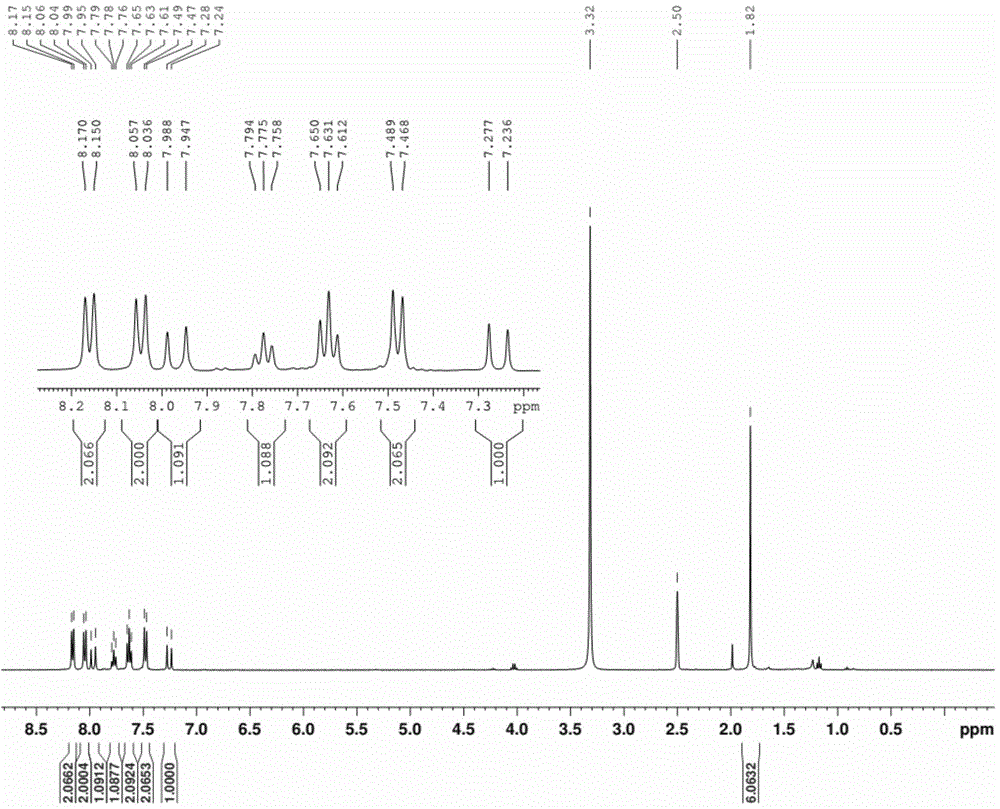
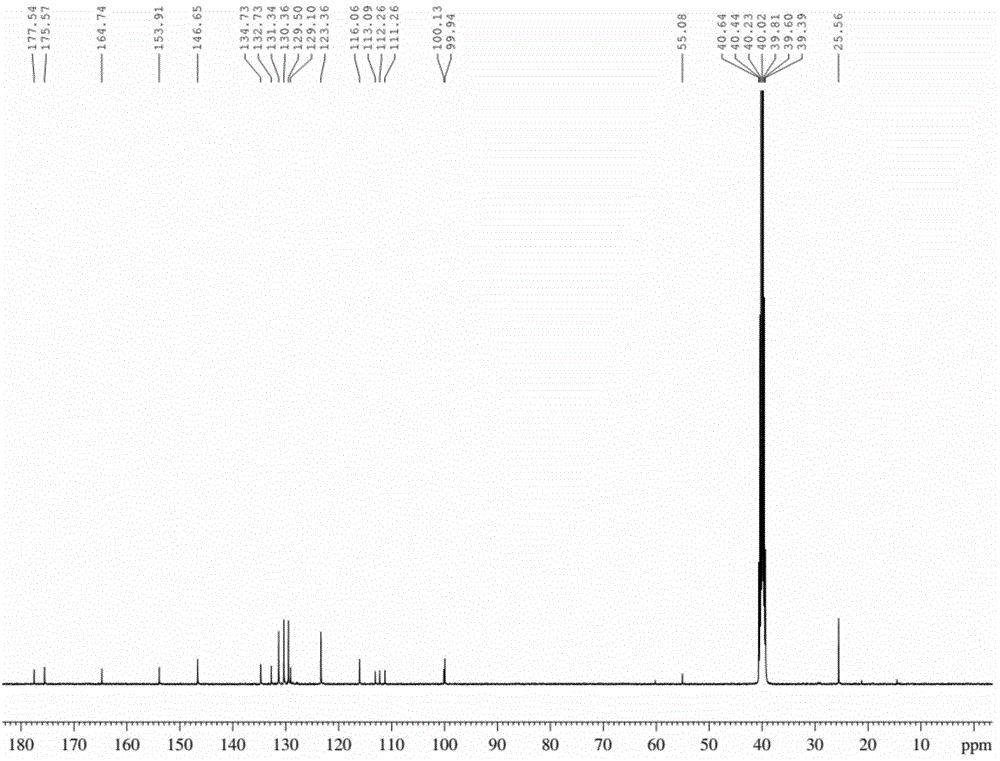
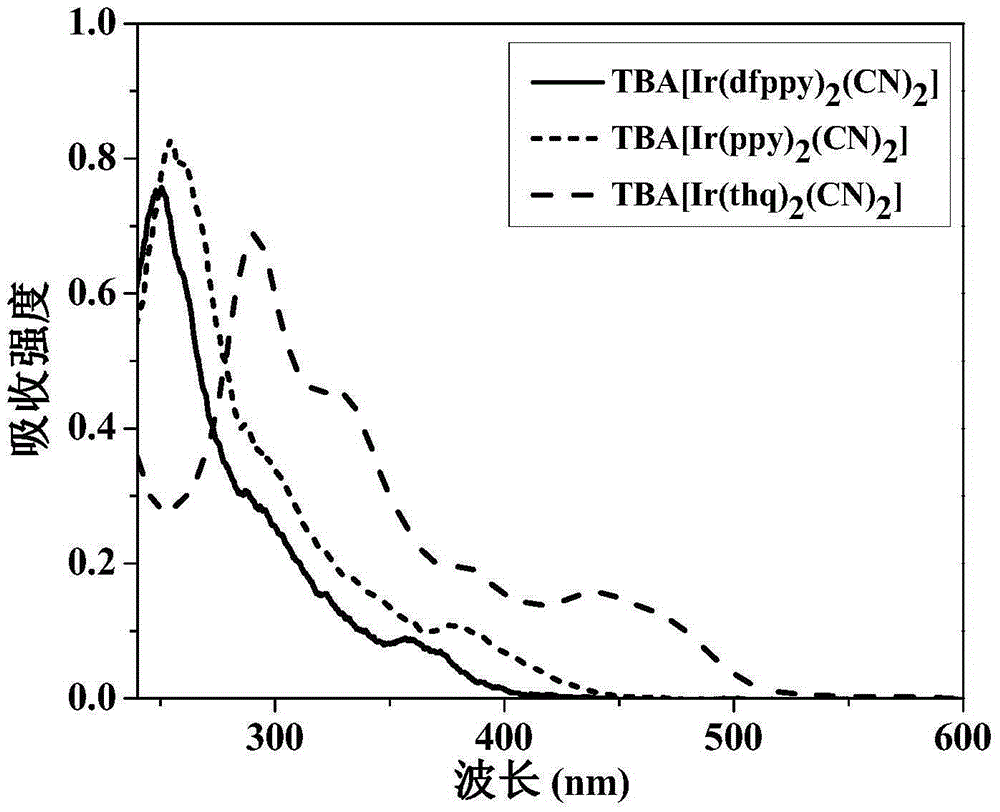
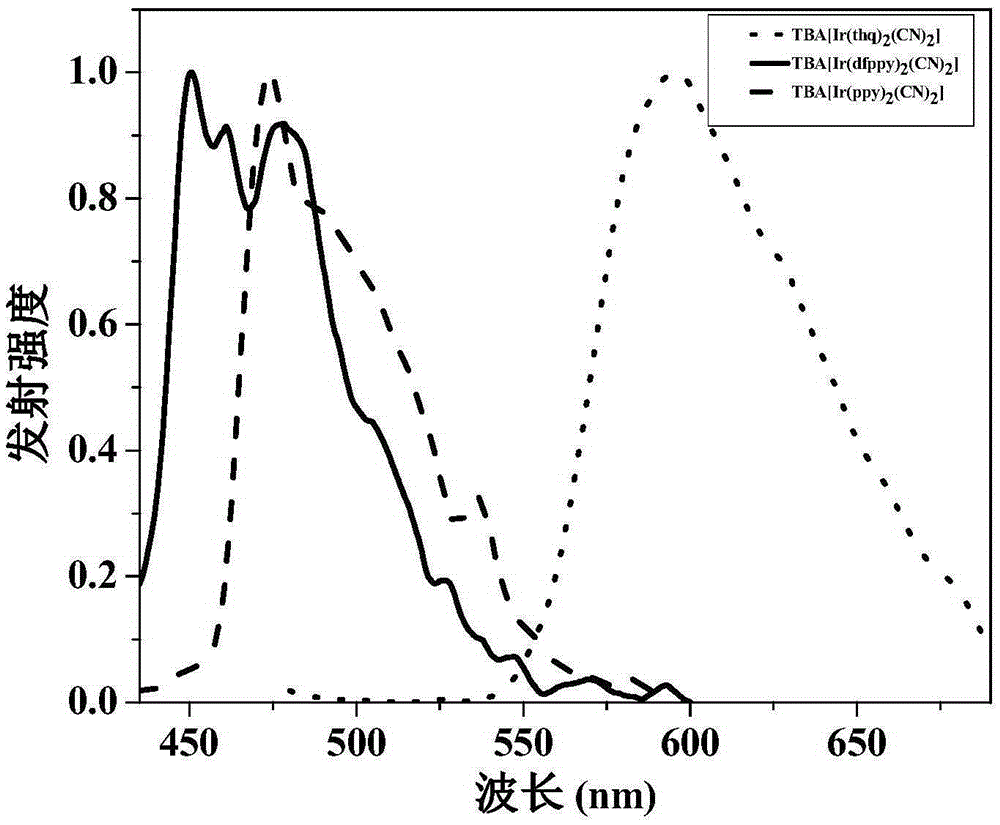
ActiveCN105294771AEasy to synthesizeHigh oxygen response sensitivityGroup 8/9/10/18 element organic compoundsFluorescence/phosphorescenceResponse sensitivityLuminous intensity
The invention discloses an anionic type iridium complex for oxygen sensing, and a preparation method and the application of the anionic type iridium complex. The anionic type iridium complex can be expressed by a general formula: [Ir(C-N)2(CN)2]-nBuN+, wherein a counter ion is a tetrabutyl ammonium ion nBuN+; a C-N ligand is selected from 2-phenylpyridine (ppy), 2,4-difluoro phenylpyridine (dfppy), 2-thiophene-quinoline (thq), 2-phenyl-quinoline (bqu) and 1-phenylisoquinoline (piq), and a molecular structure formula of the C-N ligand is as shown in the description. A preparation process of the anionic type iridium complex is simple and is easy to operate. The anionic type iridium complex can be used in the field of the oxygen sensing and has higher response sensitivity on oxygen and better selectivity; the anionic type iridium complex can also be used in the field of bio-sensing, can be used for carrying out oxygen detection in a cell or a living body and has good biocompatibility; the anionic type iridium complex has the characteristics of long phosphorescence lifetime, large Stokes shift, high light stability and the like, has relatively-sensitive luminous intensity on the oxygen and is a good oxygen probe.
Owner:NANJING UNIV OF POSTS & TELECOMM
Fluorescent microscopic counting method for detecting number of bacteria in water body
InactiveCN104593475AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementStainingCellulose acetate
The invention discloses a fade-resistant agent used in bacteria counting by virtue of a fluorescent microscopy and a fluorescent microscopic counting method for detecting the number of bacteria in a water body and belongs to the field of environmental microbiology. The fade-resistant agent is obtained by dissolving p-phenylenediamine, sodium chloride, disodium hydrogen phosphate, Tris, glycine and glycerol in deionized water and adjusting the pH. The fluorescent microscopic counting method for detecting the number of bacteria in the water body comprises the following steps: adding a fluorescent dye into a water sample, staining, adding a fade-resistant agent working liquid for carrying out fade-resistant treatment, carrying out suction-filtration on the sample subjected to the fade-resistant treatment with a cellulose acetate filtration membrane and observing under the fluorescent microscope. The method for detecting the number of bacteria in the water body is time-saving and effort-saving, low in cost and strong in fade-resistant capability and is free of background fluorescence interference and the number of bacteria in the water sample can be quickly and accurately detected.
Owner:武汉市环境保护科学研究院
Live animal two-photon excitation time-lapse detection fluorescence imaging analysis method and equipment
ActiveCN105891170BLarge imaging analysis depthImprove reliabilityDiagnostics using fluorescence emissionSensorsImaging analysisFluorescent imaging
The invention discloses a two-photon excitation time-lapse detection fluorescence imaging analysis method and equipment for living animals, which are used for analyzing the distribution and fluorescence intensity of fluorescent nano-probes (or fluorescent nano-drug-carrying analog probes) in tissues and organs in animals. Or the kinetic properties of the probe concentration changing with time, using red light or near-infrared pulsed laser two-photon to excite fluorescent nanoprobes in vivo to emit light, and detecting the fluorescence intensity of specific parts in vivo and its dynamic properties with time by time-lapse detection fluorescence imaging , has the advantages of large imaging analysis depth, high reliability and high detection sensitivity.
Owner:PEKING UNIV +1
Near infrared fluorescence probe substrate of carboxylesterase2 and application of substrate
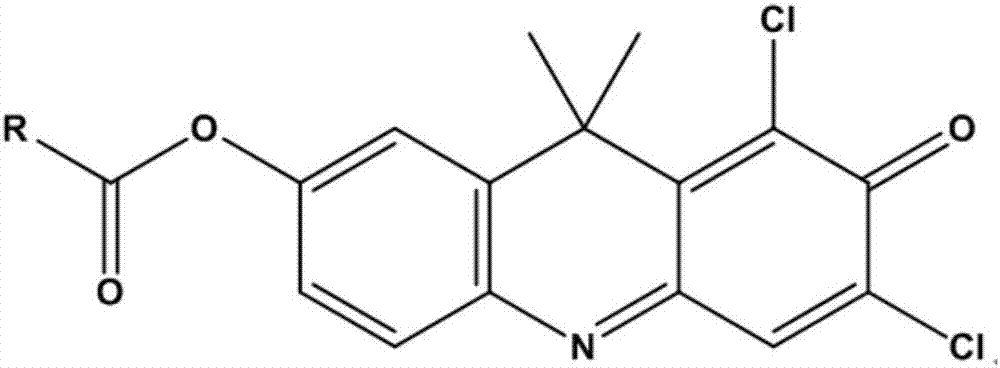
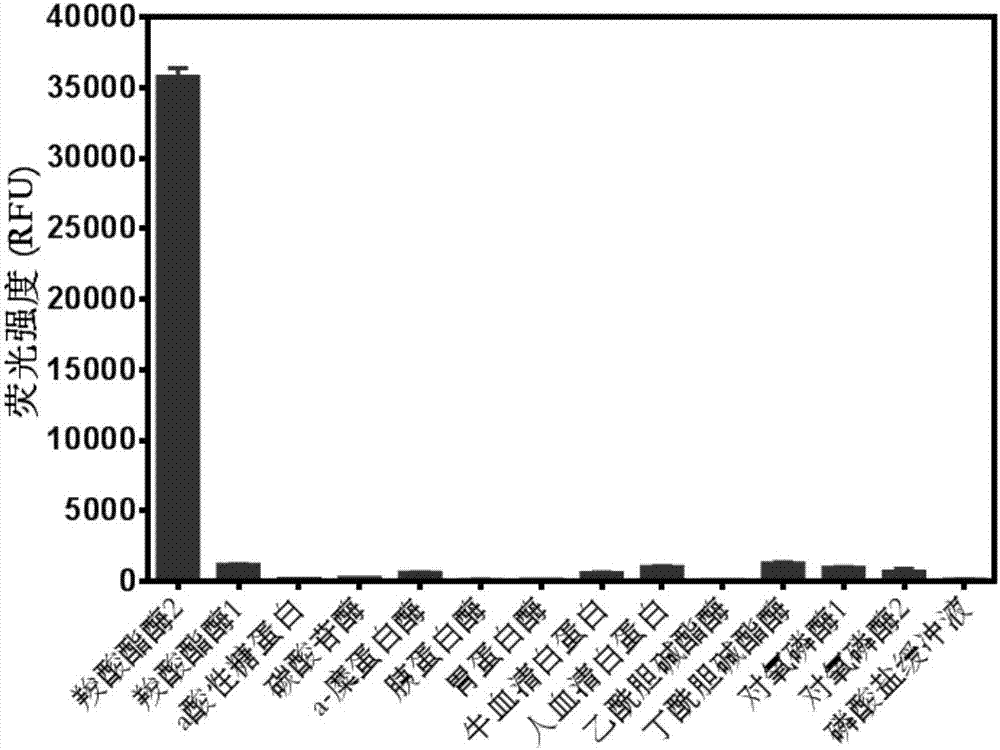
InactiveCN107089947AThe synthesis process is simpleReduce autobackground fluorescenceOrganic chemistryMicrobiological testing/measurementMetaboliteLength wave
The invention provides a near infrared fluorescence probe substrate of carboxylesterase2 and application of the substrate. The near infrared fluorescence probe substrate is aromatic ester (DDAE for short) of 1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridinone (DDAO for short). The substrate can be used for rapidly and quantitatively detecting the activity of carboxylesterase2 (CE2) and rapidly screening and evaluating carboxylesterase2 inhibitor and activator. The process of rapidly and quantitatively detecting the activity of carboxylesterase2 includes the following steps of selecting a DDAE hydrolysis reaction as a probe reaction, adding the suitable probe substrate into a buffer solution system containing carboxylesterase2, and measuring the real activity of carboxylesterase2 in various in-vitro biological samples in a linear reaction interval through the generation amount of hydrolytic metabolite DDAO in the quantitative detection unit time. The probe has good selectivity, the fluorescence emission wavelength (662 nm) of the hydrolytic metabolite DDAO is in the near infrared area, background fluorescence of the biological samples can be remarkably reduced, sensitivity is improved, and the substrate has good practicability and application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for identifying production place of turmeric essential oil based on Raman spectrum technology
ActiveCN112485238ALow costReduce background fluorescenceRaman scatteringPhysical chemistryEngineering
The invention discloses a method for identifying the production place of turmeric essential oil based on a Raman spectrum technology. The method uses qualitative and quantitative identification methods at the same time, and comprises the following specific steps: (1) collecting a calibration set verification set and processing Raman spectrum data; (2) establishing a judgment model: (2.1) establishing a PCA qualitative judgment model, and (2.2) establishing a PLS-DA quantitative judgment model; and (3) measuring the sample to be measured. The method has the characteristics of small identification error, high identification speed and no damage.
Owner:珠海市亚波光子检测有限责任公司
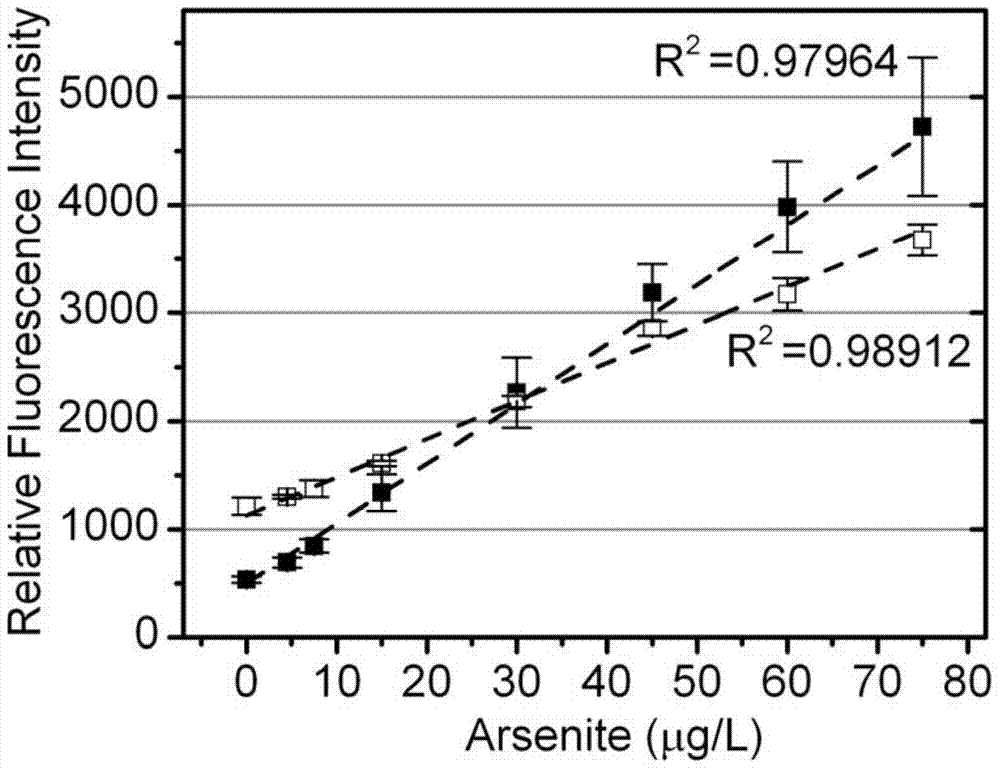
Arsenic induced operon gene and application thereof
ActiveCN104745582AHigh sensitivitySpecific detectionBacteriaMicrobiological testing/measurementNucleotideWild type
The invention discloses an arsenic induced operon gene and application thereof. The operon gene has a nucleotide sequence represented by SEQ ID NO:1 or a nucleotide sequence complementary with the nucleotide sequence represented by SEQ ID NO:1. An arsenic induced operon is a muton of a wild type arsenic induced operon and can be directly used for optimally constructing an arsenic induced biosensor and detecting the content of arsenic. An arsenic induced bacteria biological sensor constructed based on the operon has the advantages of being high in sensitivity, specific in arsenic detection and low in background fluorescence.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Two-photon fluorescent probe for double detection of sulfur dioxide and viscosity and preparation of two-photon fluorescent probe
InactiveCN114149359AReduce background fluorescenceImprove response rateOrganic chemistryFluorescence/phosphorescenceFluoProbesChemical physics
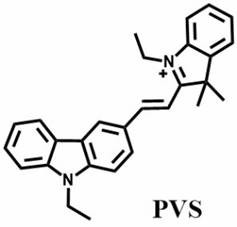
The invention discloses a two-photon fluorescent probe for sulfur dioxide and viscosity dual detection and a preparation method of the two-photon fluorescent probe. The compound structure of the fluorescent probe is shown as a compound PVS. The fluorescent probe realizes two-photon excitation response to viscosity by utilizing a twisted intramolecular charge transfer (TICT) mechanism between indole salt and carbazole. Meanwhile, the carbon-carbon double bond connected with the indole salt can realize response to sulfur dioxide through nucleophilic addition reaction. The PVS has the advantages that the PVS can track the fluctuation of sulfur dioxide and viscosity at the same time, and has two emissions which are easy to distinguish. The PVS can also accurately visualize the form of the mitochondria, and the real-time accurate monitoring of the sulfur dioxide and the viscosity in the mitochondria under the excitation of the two photons is successfully realized.
Owner:UNIV OF JINAN
Weak background fluorescence type resin embedding method
ActiveCN103808553AReduce background fluorescenceLow costPreparing sample for investigationBiological systems engineeringBackground fluorescence
The invention discloses a weak background fluorescence type resin embedding method and belongs to the technical field of biological engineering. In the method, a fluorescence biological sample is permeated and embedded by using a resin solution in which Sudan black B is dissolved as a dying and embedding solution. On the premise that a biological sample obtained by adopting the method keeps the fluorescence signal intensity, the background fluorescence is greatly reduced. The method can be applicable to a large sample with a centimeter size; the obtained biological sample can be used for continuously slicing in an ultrathin manner. The method provided by the invention has the advantages of being low in cost and simple in operation steps, and is applicable to popularization and application in ordinary laboratories.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method of levodopa nanoparticles and biosensing application of levodopa nanoparticles
ActiveCN112179879AThe synthesis process is simple and environmentally friendlyGood biocompatibilityMicrobiological testing/measurementFluorescence/phosphorescenceFluoProbesAdenosine
The invention relates to a preparation method of levodopa nanoparticles and biosensing application of the levodopa nanoparticles, and belongs to the technical field of nano biosensing. Levodopa (L-DA)is used as a unique precursor to synthesize levodopa nanoparticles (LNDS) at room temperature, and the formed LNDS can quickly and effectively quench a dye-labeled oligonucleotide fluorescent probe (FAM-DNA) through electrostatic interaction. Complementary deoxyribonucleic acid (cDNA) or adenosine triphosphate (ATP) is competitively combined with a fluorescent probe, so that fluorescence is recovered, and an effective sensing platform for detecting biomolecules is constructed. In addition, the sensing platform can also perform high-sensitivity and selective detection on adenosine triphosphatein a complex system (human serum). The synthetic process of the levodopa nanoparticles is simple and environmentally friendly, the toxic and side effects on cells and organisms are low, the levodopananoparticles have a very strong quenching effect on dye-labeled oligonucleotides, the background fluorescence is greatly reduced, and the signal-to-noise ratio is increased.
Owner:NANJING UNIV OF TECH
Water-soluble red fluorescence mitochondria targeting probe and application thereof
ActiveCN112079771AImprove clarityIncrease contrastOrganic chemistryFluorescence/phosphorescenceFluoProbesStaining
The invention relates to a water-soluble red fluorescence mitochondria targeting probe and application, and belongs to the field of new materials. The invention discloses a water-soluble red fluorescence mitochondria targeting probe, which has a structural general formula shown in the specification, wherein M is an aromatic ring, and N is a hydrophilic group. Compared with other commercial mitochondrial fluorescence probes, the targeting probe provided by the invention has relatively good water solubility. When the fluorescent probe disclosed by the invention is used for dyeing cells, mitochondria can be targeted; the probe has higher biocompatibility and almost has no background fluorescence, and the weak scattered light and autofluorescence of a biological sample at a red fluorescence waveband are beneficial to reducing background signals, so that the probe has a high signal-to-noise ratio. In addition, the red fluorescence has better penetration depth, has more outstanding advantages when being used for mitochondrial imaging of cells, can be used for observing the mitochondrial imaging process of the cells in situ under the rinsing-free condition, and can track the state of themitochondria of the cells for a long time.
Owner:HUAZHONG UNIV OF SCI & TECH
Graphene oxide-containing amplification system and application of system in detection of colorectal cancer markers
PendingCN110819717AReduce testing costsEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceOncologyBiochemistry
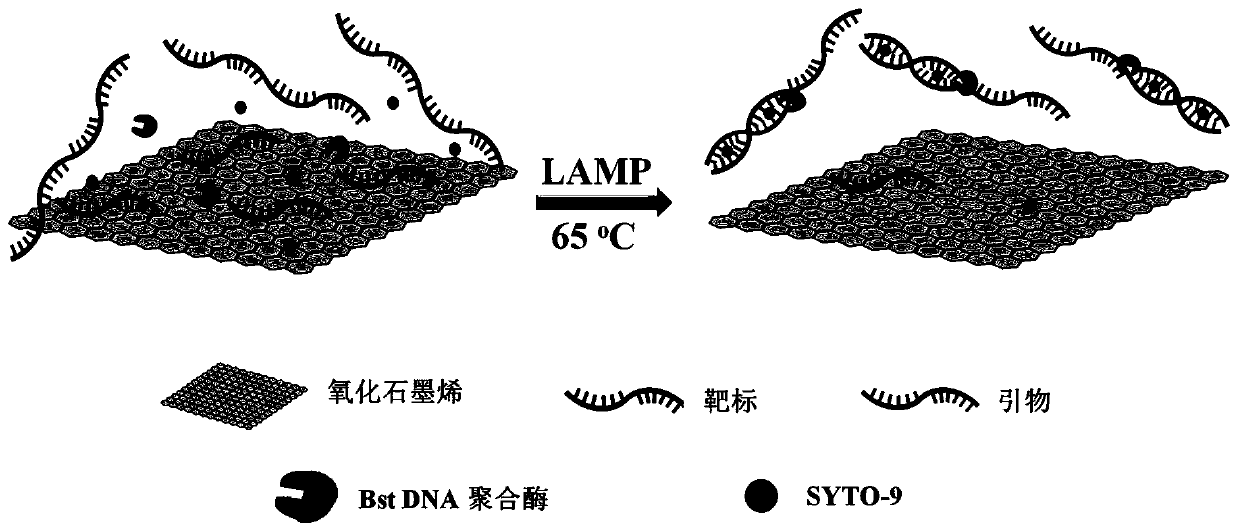
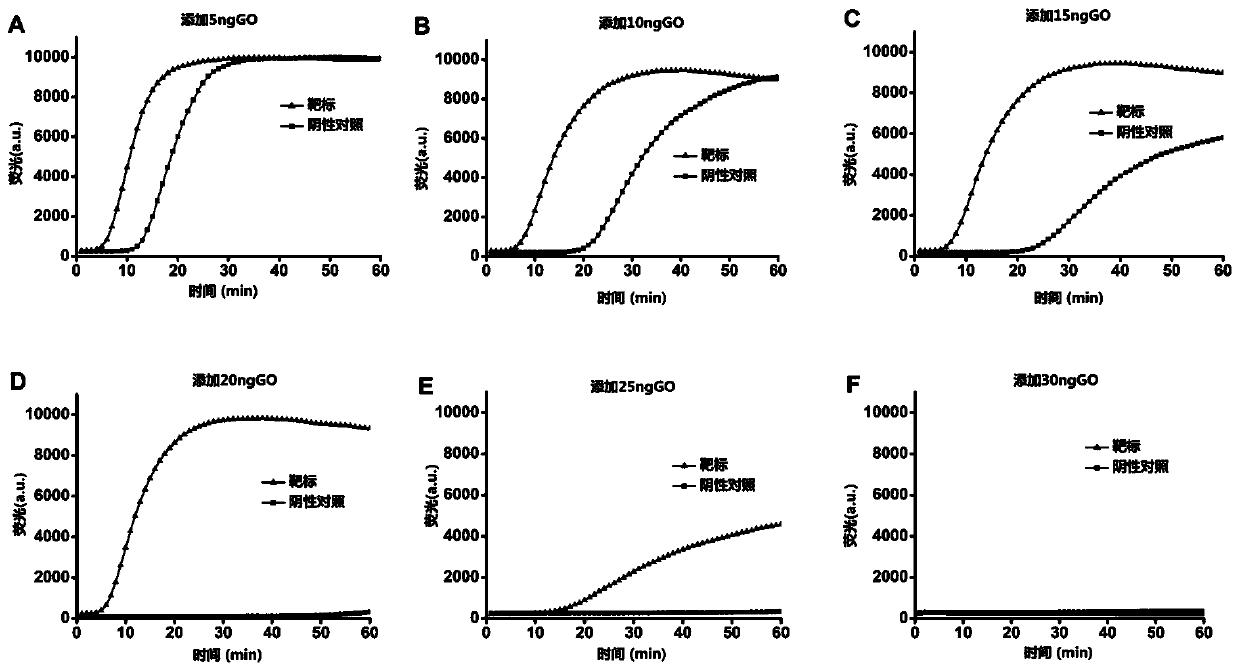
The present invention discloses a graphene oxide-containing amplification system and an application of the system in detection of colorectal cancer markers. The amplification system comprises an isothermal amplification buffer, MgSO4, a dNTP mixture, a target-specific primer set, DNA polymerase, an amplification template, and 5-30 ng of graphene oxide. A mechanism of graphene oxide inhibiting non-specific amplification in LAMP is verified, the optimal amount of the graphene oxide in a LAMP amplification system is optimized, and the amplification system is applied in the detection of rectal cancer markers.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD +1
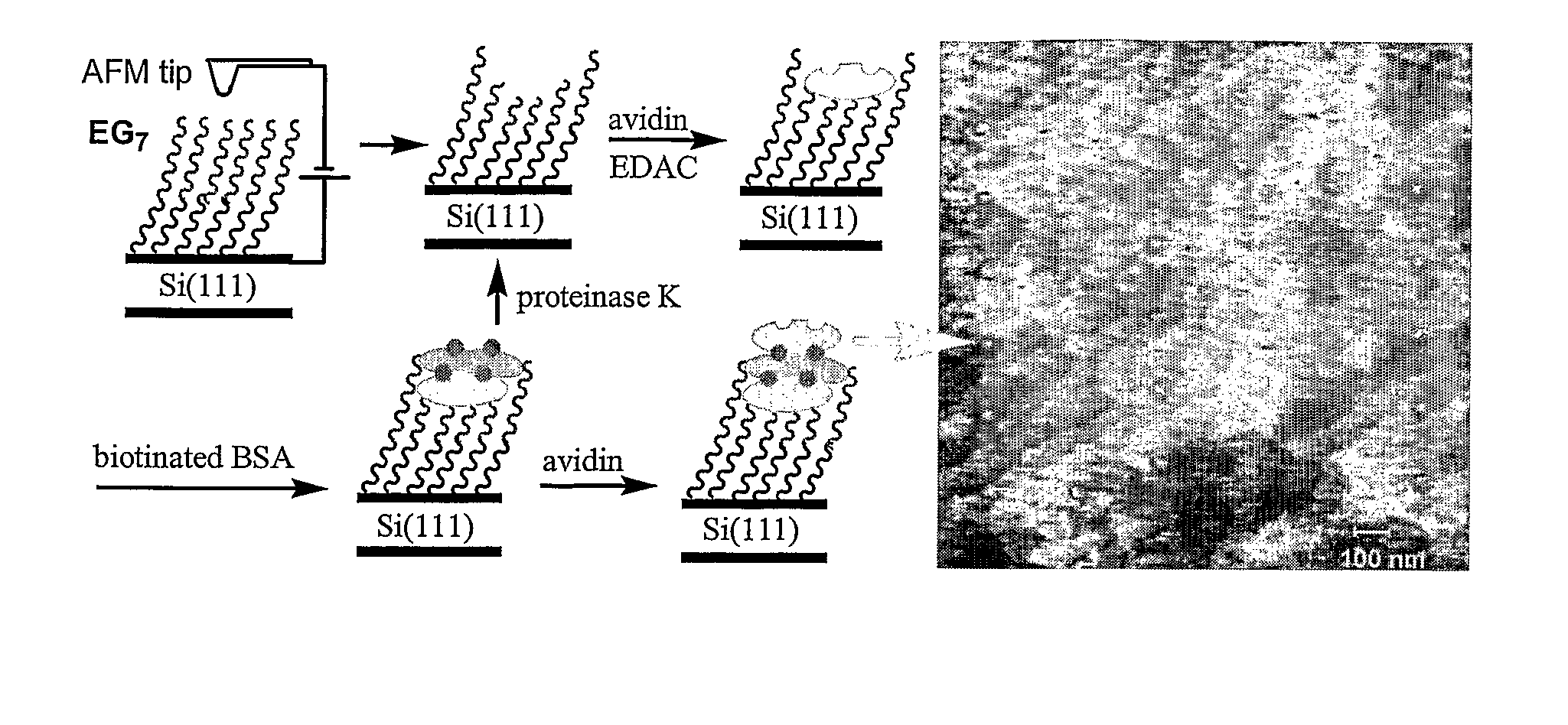
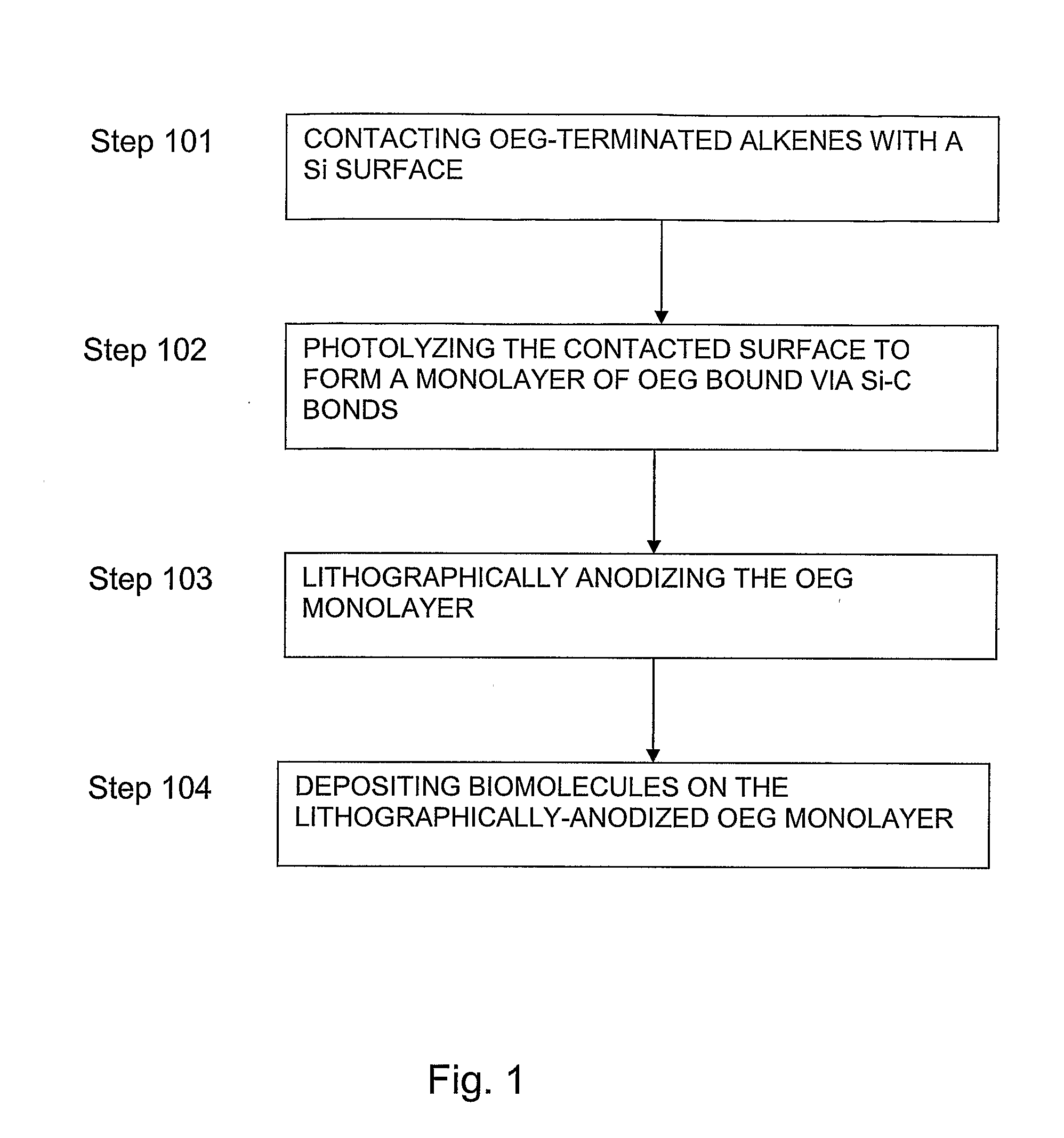
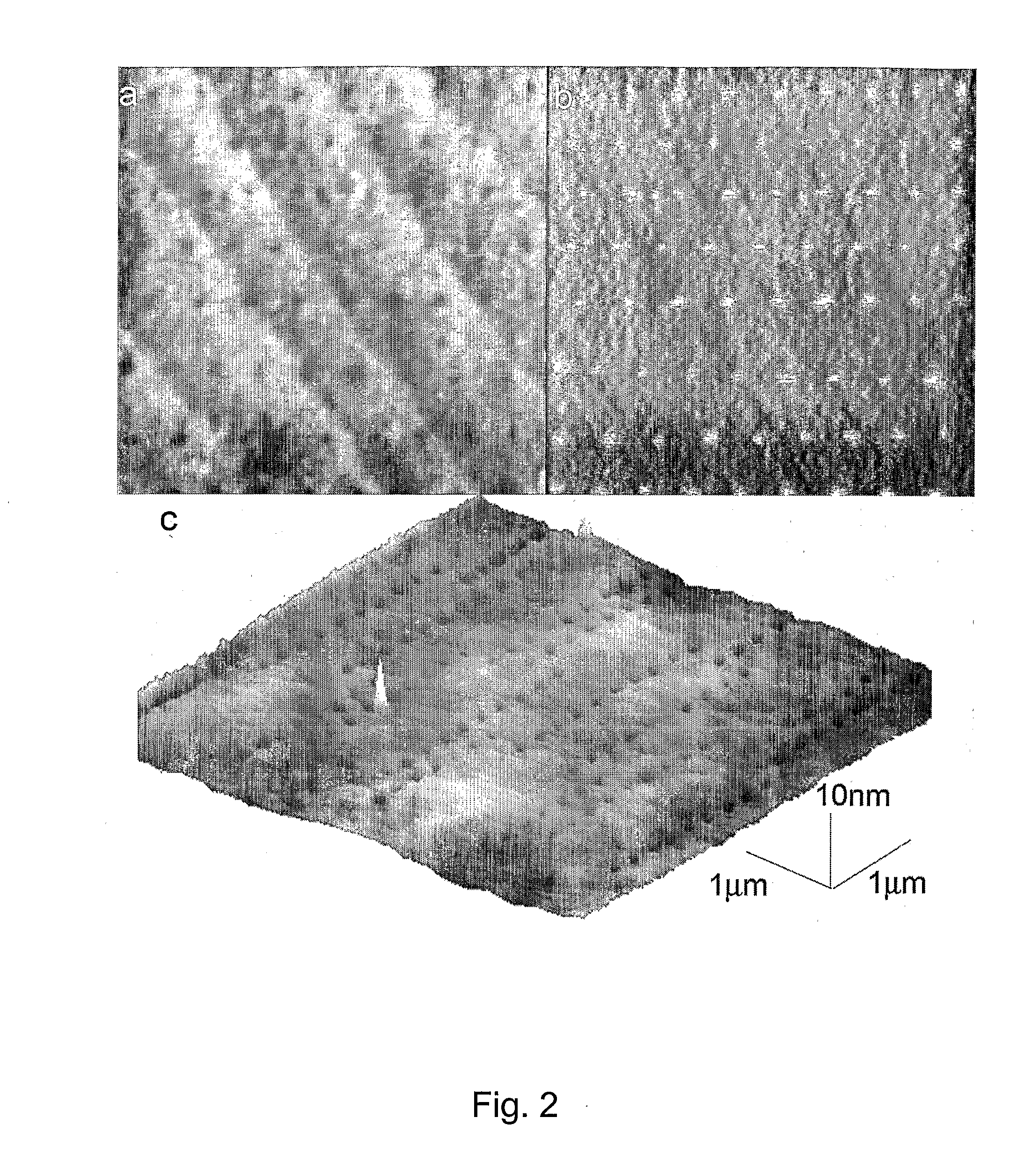
Preparation of Nanometric Arrays of Biomolecules on Oligo-or Poly(Ethylene Glycol) Films on Silicon Surfaces
ActiveUS20070212555A1High detection sensitivityIncrease probe densityBioreactor/fermenter combinationsMaterial nanotechnologyCrystallographyPolyethylene glycol
The present invention is generally directed to nanometric biomolecular arrays and to a novel approaches for the preparation of such nanoarrays, based on binding of biomolecules, such as avidin, to templates generated by lithographically-an-odizing biocompatible ultrathin films on silicon substrates using AFM anodization lithography. The present invention is also directed to methods of using such arrays.
Owner:UNIV HOUSTON SYST
Dual-wavelength excitation type feedback phototherapeutic instrument
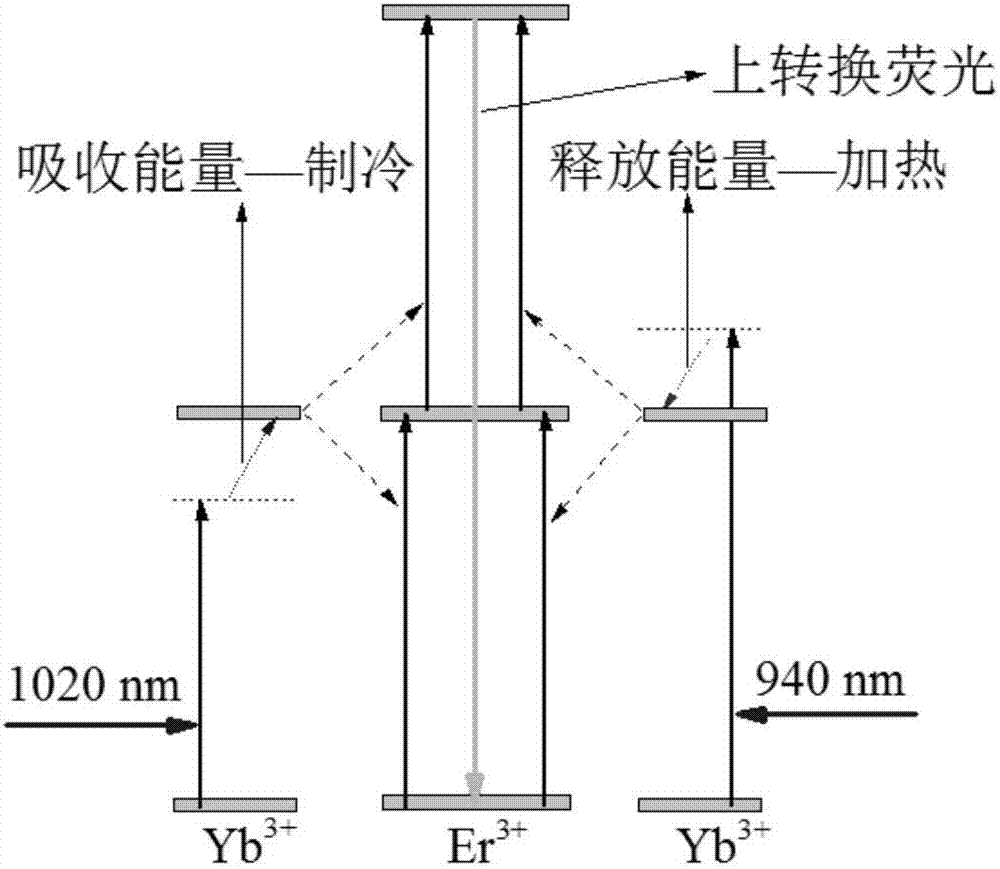
ActiveCN107440795AImprove stabilityIncrease penetration depthPolycrystalline material growthFrom normal temperature solutionsOnline interventionFluorescence
The invention relates to phototherapeutic instruments, in particular to a dual-wavelength excitation type feedback phototherapeutic instrument and aims to solve technical problems of harms to normal cells, lack of a noninvasive temperature feedback function and long treatment period of an existing phototherapeutic instrument. The phototherapeutic instrument comprises a double-channel signal generator, a heating laser, a refrigerating laser, an optical fiber transmission system, an up-conversion thermotherapy probe, a spectrograph and a computer. The optical fiber transmission system comprises a coupler, an annular and a focus mirror in sequential connection through optical fibers. Two pulse laser beams in different wavelengths are used for heating and refrigerating respectively; by up-conversion fluorescence signal resolution of the probe temperature, temperature feedback in a phototherapeutic process is realized, and input laser parameters are further adjusted according to feedback temperature to realize online intervention. The dual-wavelength excitation type feedback phototherapeutic instrument is simple in operation and capable of inhibiting overheating and shortening treatment cycle.
Owner:哈尔滨凯美斯科技有限公司
Design method of primers and probes for detecting pathogens
PendingCN109554506AReduce usageGuaranteed SensitivityMicrobiological testing/measurementMicroorganism based processesTrue positive rateDesign methods
The invention provides a design method of primers and probes for detecting multiple subtypes of pathogens such as high-risk HPV. The designed primers and probes can be used for preliminary detection and type identification of multiple subtypes of the pathogens that is possible to exist in samples. Based on the affinity of multiple subtypes of the pathogens such as high-risk HPV in evolutionary trees, the primers and probes for multiple subtypes of the pathogens are designed, background fluorescence value can be significantly reduced, detection flux is obviously increased and the detection costis greatly reduced while detection accuracy, sensitivity and specificity are ensured.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Mycoplasma pneumoniae rapid detection primer group and kit
InactiveCN109735640AImprove accuracyImprove adaptabilityMicroorganismsMicrobiological testing/measurementNucleic acid amplification techniqueReaction tube
The invention relates to a mycoplasma pneumoniae (MP) rapid detection primer group and a kit. The mycoplasma pneumoniae rapid detection primer group comprises an upper stream primer, a lower stream primer and a probe which detect a mycoplasma pneumoniae gene group P1 gene sequence. The kit comprises a lysate solution, a reconstitution fluid, an EMA reaction tube containing primer probes, a positive quality control product and a negative quality control product. The primer group can be specifically combined with the mycoplasma pneumoniae P1 gene, and cooperate with EMA (Enzymes Mediated Amplification, a multienzyme mediated nucleic acid amplification technology, namely a normal temperature nucleic acid amplification technology) to prepare the mycoplasma pneumoniae detection kit, and the mycoplasma pneumoniae rapid detection primer group and kit have the advantages of easy to operate, short in consumed time, high in sensitivity and specificity and the like.
Owner:苏州晶睿生物科技有限公司
Two-photon excitation delay detection fluorescence imaging analysis method and equipment for live animal
ActiveCN109142305ALarge imaging analysis depthImprove reliabilityDiagnostics using fluorescence emissionSensorsPhotonFluorescence intensity
The invention discloses a two-photon excitation delay detection fluorescence imaging analysis method and equipment for a live animal. The method is used for analyzing the time-varying kinetic propertyof distribution, fluorescence intensity or a probe concentration of nano fluorescence probes (or fluorescence nano drug carrier simulated probes) in a tissue and an organ in an animal body; the in-vivo fluorescence nano probes are subjected to two-photon excitation by red light or near infrared pulse laser, so as to perform delay detection on the fluorescence intensity and the time-varying kinetic property of an in-vivo specific part by a fluorescence imaging mode; the method has the advantages of large imaging analysis depth, high reliability, high detection sensitivity and the like.
Owner:PEKING UNIV +1
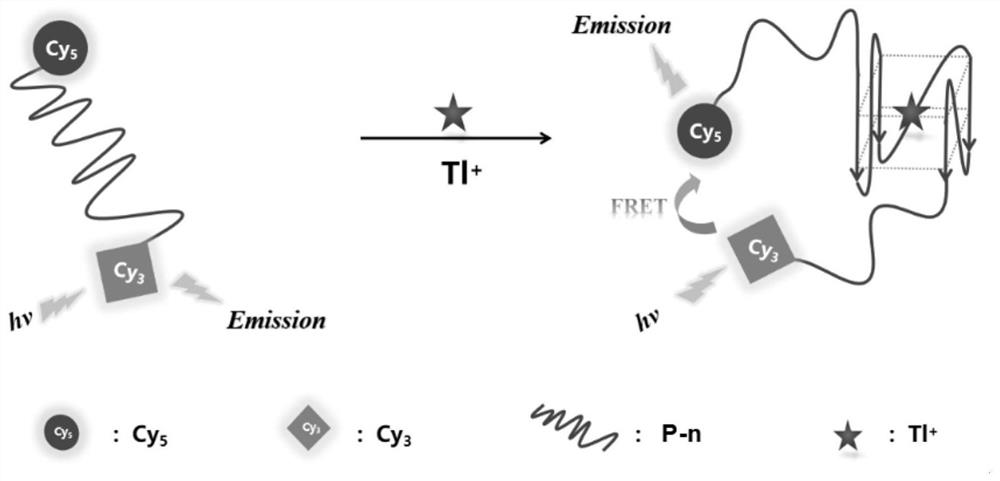
Fluorescent probe for detecting Tl<+> based on nucleic acid aptamer
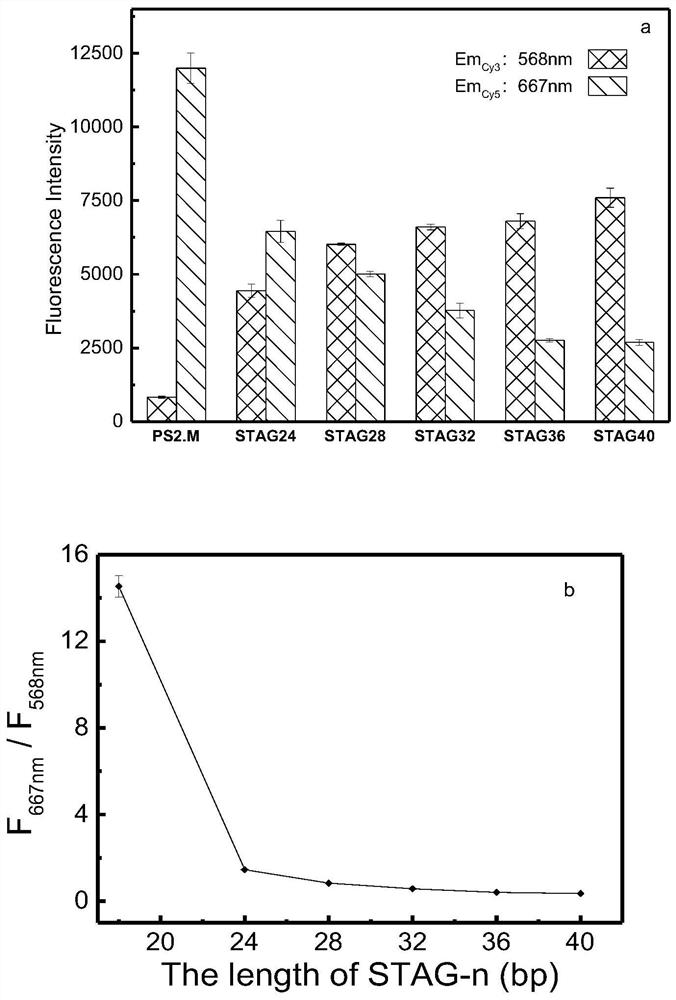
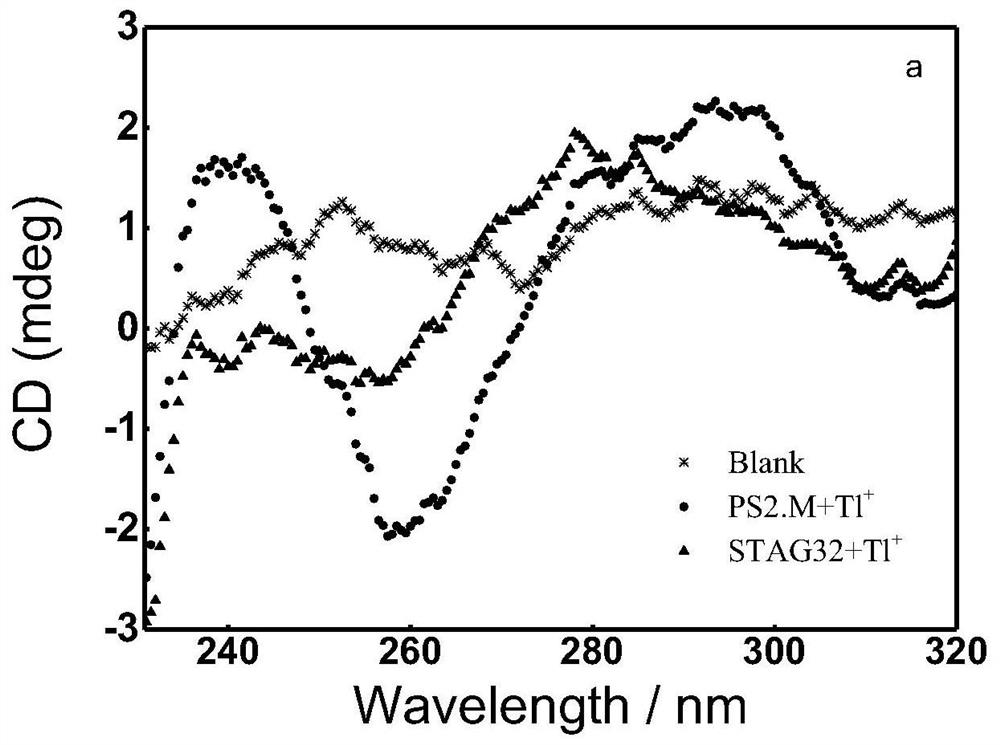
ActiveCN113237856ASpecific captureReduced fluorescence energy transfer efficiencyBiological testingFluorescence/phosphorescenceAptamerFluoProbes
The invention belongs to the technical field of chemical analysis and detection, and aims at solving problems that in recent years, thallium environment detection and poisoning identification and troubleshooting requirements are met. Portable detection means are lacked, a G-rich nucleic acid aptamer fragment capable of specifically capturing Tl<+> is designed, and a double-label fluorescence technology and a fluorescence resonance energy transfer principle are combined, the fluorescent probe for detecting Tl<+> based on the nucleic acid aptamer is constructed. The nucleic acid aptamer is obtained by screening and optimizing a G-rich nucleic acid aptamer by using a Poly-T extension strategy, and is named as GACT32. cyanine dyes Cy3 and Cy5 are adopted as fluorescence signal indicating groups to be modified at the 5' end and the 3' end of the nucleic acid aptamer respectively. According to the probe, background fluorescence of a traditional fluorescent probe is greatly reduced, interference of K<+>, Na<+> and other ions in a solution is eliminated, specific detection of Tl<+> is achieved, the linear range is 10 mu M-10 mM, and a portable detection method is provided for thallium ion environment detection and poisoning investigation.
Owner:ACADEMY OF MILITARY MEDICAL SCI
HOCl fluorescent probe based on pyronin hydrazine, preparation method and application
ActiveCN112694469AReduce background fluorescenceFast fluorescent off-on responseOrganic chemistryFluorescence/phosphorescenceBackground fluorescenceFluoProbes
The invention provides an HOCl fluorescent probe based on pyronin hydrazine, a preparation method and application. The structural formula of the probe is shown as a formula I and a formula II. According to the invention, the probe is constructed by combining a hydrazine reaction site and a pyronin fluorophore, has extremely low background fluorescence due to C=N isomerization and the PET process, provides rapid and significant fluorescence off-on response to HClO in the red light region, has extremely high sensitivity, and has been successfully used for distinguishing cancer cells / tissues from normal cells / tissues.
Owner:SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com