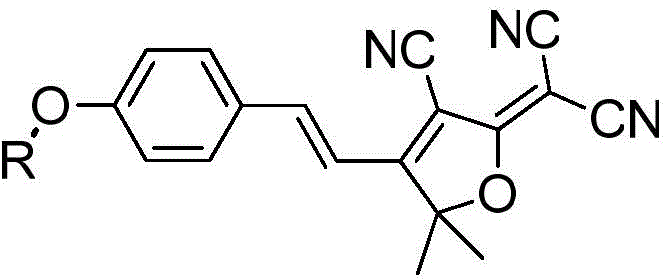
Human intestinal tract carboxylesterase activity detection fluorescent probe substrate and use thereof
A technology of human intestinal carboxylate and intestinal carboxylate, which is applied in the field of medicine to achieve high sensitivity, enhanced sensitivity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of (E)-2-(4-(4-benzoyloxystylenyl)-3-cyano-5,5-dimethylfuran-2(5H)-ylidene)malononitrile method
[0046] (1) To 10 mL of (E)-2-(4-(4-hydroxystyryl)-3-cyano-5,5-dimethylfuran-2(5H)-ylidene) containing 0.5 mmol In the N,N dimethylformamide solution of malononitrile and 0.625mmol triethylamine, slowly add 0.6mmol of p-benzoyl chloride (dissolved in 5mL of N,N dimethylformamide) dropwise, and control the temperature at 0℃;
[0047] (2) After stirring in an ice bath for 1 h, the solution was naturally warmed to room temperature and stirred overnight;
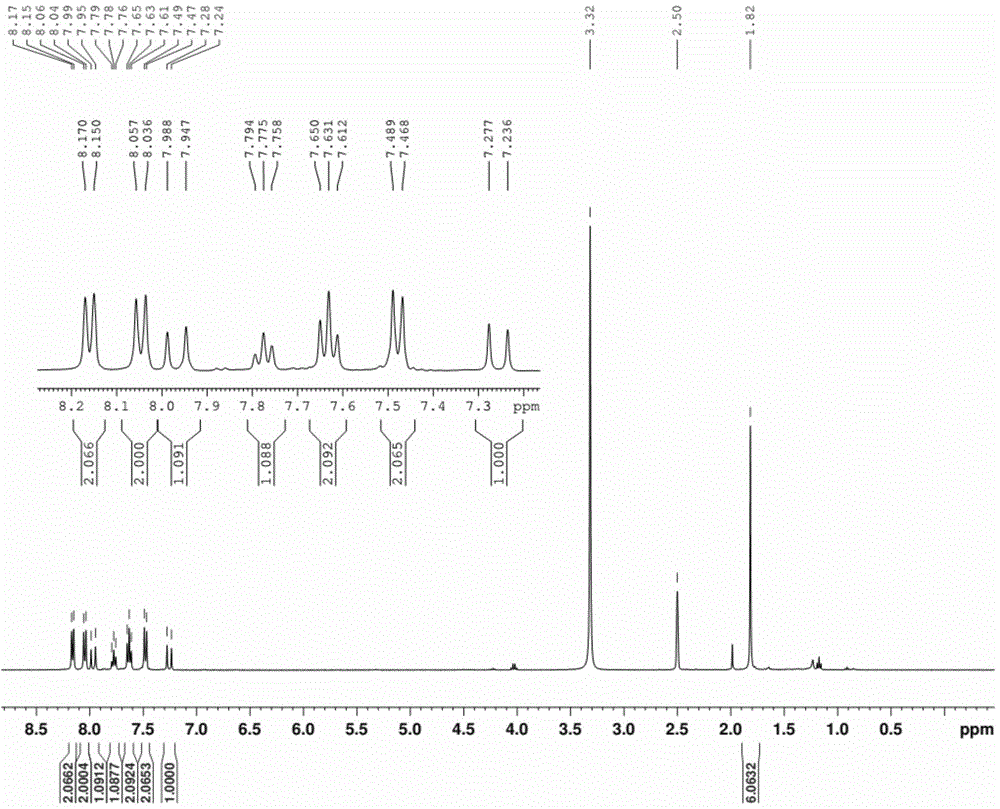
[0048] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1:3, v / v) to obtain 30.6 mg of orange solid powder (synthesized See the route Figure 9 ). 1 HNMR(400MHz,DMSO)δ8.16(d,J=7.9Hz,2H),8.05(d,J=8.5Hz,2H),7.97(d,J=16.5Hz,1H),7.78(t,J= 7.2Hz, 1H), 7.63(t, J=7.7Hz, 2H), 7.48(d, J...
Embodiment 2
[0050] Selectivity in recombinantly expressed human single enzymes
[0051] (1) Prepare 99 μL metabolic reaction system in advance, including pH7.4 PBS buffer (10 mM), recombinantly expressed human hCE1 (10 μg / mL) / human hCE2 (10 μg / mL) / serum albumin (10 μg / mL) / Acetylcholinesterase (10μg / mL) / butyrylcholinesterase (1.5U / L) / paraoxonase 1 (10μg / mL) / paraoxonase 2 (10μg / mL) / phosphate buffer at 37 Pre-incubate with shaking at ℃ for 10 minutes;
[0052] (2) Add 1 μL of (E)-2-(4-(4-benzoyloxystyryl)-3-cyano-5,5-dimethylfuran with a final concentration of 20 μM to the reaction system -2(5H)-ylidene)malononitrile starting reaction;
[0053] (3) After 30 minutes, 100 μL of ice acetonitrile was added, and the reaction was terminated after vigorous shaking;
[0054] (4) Fluorescence detection (E x =560nm, E m =612nm); calculate the fluorescence intensity in each system (see Figure 5 ).
Embodiment 3
[0056] Protein concentration of hCE2-catalyzed linear reactions in recombinant single enzymes
[0057] (1) Prepare 99 μL of hCE2 metabolic reaction system in advance, including pH 7.4 PBS buffer (10 mM), recombinant human hCE2 (0-15 μg / mL), and pre-incubate with shaking at 37°C for 10 minutes;
[0058] (2) Add 1 μL of (E)-2-(4-(4-benzoyloxystyryl)-3-cyano-5,5-dimethylfuran with a final concentration of 20 μM to the reaction system -2(5H)-ylidene)malononitrile starting reaction;
[0059] (3) After 30 minutes, 100 μL of ice acetonitrile was added, and the reaction was terminated after vigorous shaking;
[0060] (4) Fluorescence detection (E x =560nm, E m =612nm); calculate the fluorescence intensity in each system (see Image 6 ); the standard curve is obtained as follows: Y=101.9*X-97.05, where Y represents the fluorescence intensity of the product, X represents the hCE2 concentration, the unit is μg / mL, and the range of X is 0-15 μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com