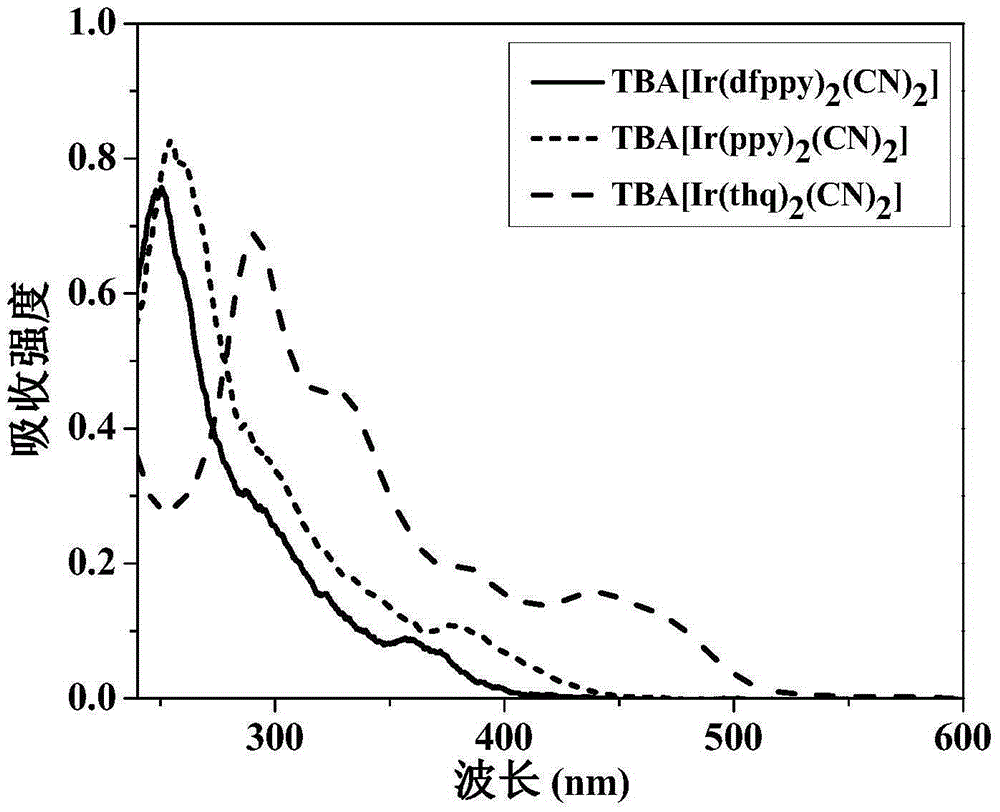
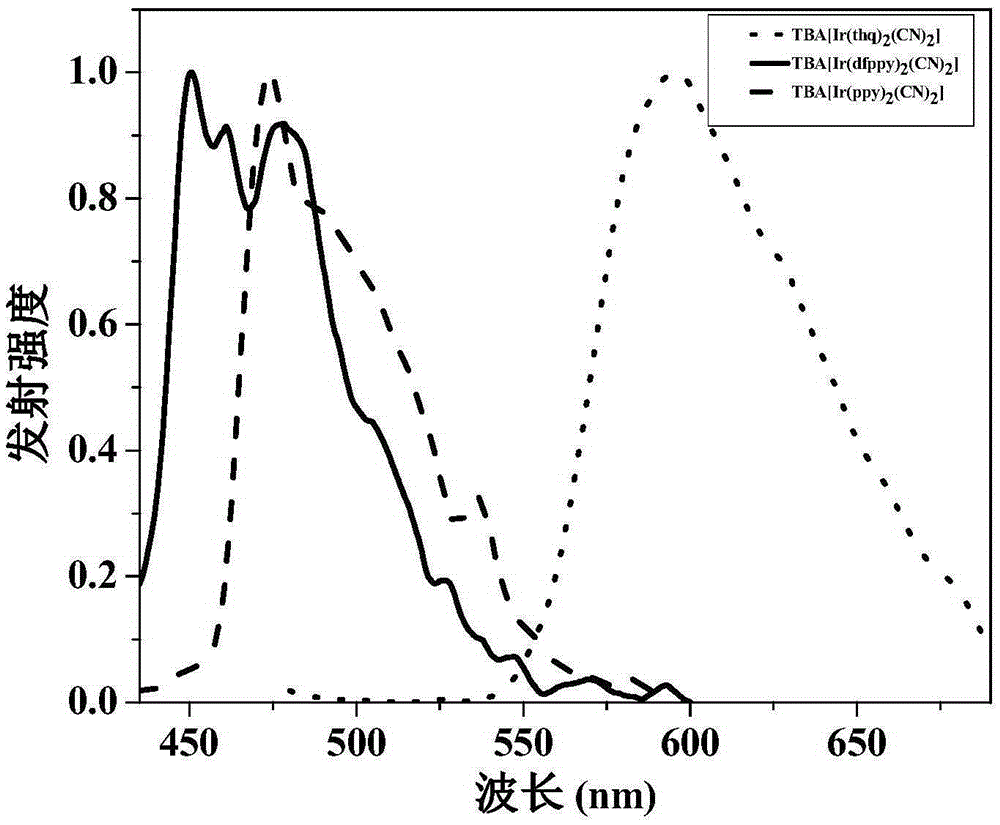
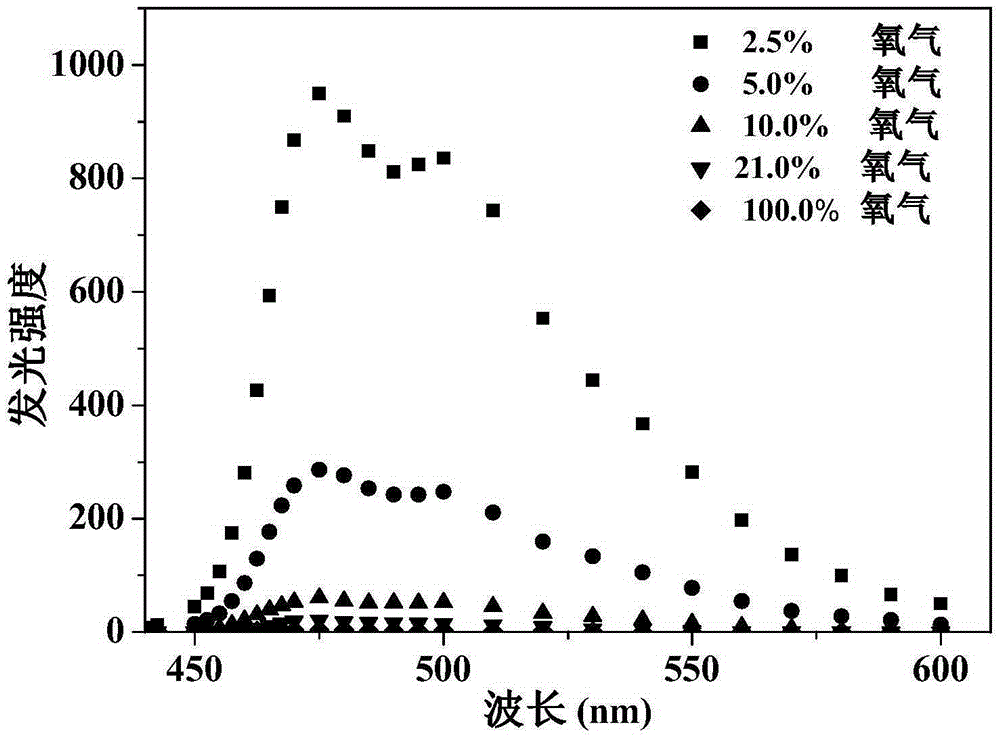
Anionic type iridium complex for oxygen sensing, and preparation method and application thereof
An iridium complex, anionic technology, applied in the field of the preparation of anionic iridium complexes, can solve the problems of insufficient stability, aggregation and quenching luminescence, synthesis difficulties, etc., and achieves rich photophysical properties, good selectivity, and synthesis. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, anionic iridium complex [Ir(dfppy) 2 (EN) 2 ] - wxya + preparation.
[0032] (1) Determine and prepare the C^N ligand. The C^N ligand is determined to be 2,4-difluorophenylpyridine (dfppy), which can be prepared according to the existing preparation methods in the prior art.
[0033] The reaction formula for preparing the monomer 2,4-difluoro-2-phenylpyridine is shown below.
[0034]
[0035] The specific process is: weigh 2-bromopyridine (1.0g, 6.37mmol), 2,4-difluorophenylboronic acid (1.03g, 6.5mmol) and tetrakis(triphenylphosphine)palladium (0.370g, 0.32mmol) and add In a 50mL two-necked bottle, vacuumize and blow nitrogen for three times, then inject toluene (9mL) and saturated K 2 CO 3(aq) (3 mL), ethanol (3 mL), reflux at 80° C. for 15 hours. Cool to room temperature, extract with dichloromethane and distilled water, combine the organic phases, and spin dry under reduced pressure to obtain 1.12 g of the product with a yield of 90%. 1 HNMR...
Embodiment 2
[0043] Embodiment 2, anion iridium complex [Ir(ppy) 2 (EN) 2 ] - wxya + Preparation of:
[0044] (1) Determine and prepare the C^N ligand, confirm that the C^N ligand is 2-phenylpyridine (ppy), which can be prepared according to the existing preparation methods in the prior art.
[0045] (2) Anionic iridium complex [Ir(ppy) 2 (EN) 2] - wxya + Preparation of:
[0046] (A) Dimer iridium complex (C^N) 2 Ir(μ-Cl) 2 Ir(C^N) 2 The synthesis of 2-phenylpyridine dichloro bridge, the specific reaction is: weigh IrCl 3 . 3H 2 O (528mg, 1.50mmol) and 2-phenylpyridine (ppy) (864mg, 4.50mmol) were added into a 50mL two-neck flask, and nitrogen was circulated three times under vacuum. Ethylene glycol ether (15 mL) and distilled water (3 mL) were injected with a syringe, the reaction temperature was 100° C., and the mixture was stirred under reflux for 36 hours under a nitrogen atmosphere until a precipitate formed. After stopping the reaction, cool to room temperature, filter...
Embodiment 3
[0049] Embodiment 3, anion iridium complex [Ir(thq) 2 (EN) 2 ] - wxya + preparation.
[0050] (1) Determine and prepare the C^N ligand, confirm that the C^N ligand is 2-thienyl quinoline (thq), which can be prepared according to the existing preparation methods in the prior art.
[0051] (2) Anionic iridium complexes [Ir(thq) 2 (CN) 2 ] - wxya + Preparation of:
[0052] (A) Dimer iridium complex (C^N) 2 Ir(μ-Cl) 2 Ir(C^N) 2 The synthesis of, that is, the synthesis of 2-thiophene-quinoline dichloro bridge, its specific synthesis method is: weigh IrCl 3 . 3H 2 O (500mg, 1.42mmol) and 2-thiophene-quinoline (thq) (600.0mg, 2.84mmol) were added into a 50mL two-neck flask, and vacuum pumped and nitrogen was circulated three times. Ethylene glycol ether (12 mL) and distilled water (4 mL) were injected with a syringe, the reaction temperature was 120° C., and the mixture was refluxed and stirred for 36 hours under a nitrogen atmosphere until a precipitate formed. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com