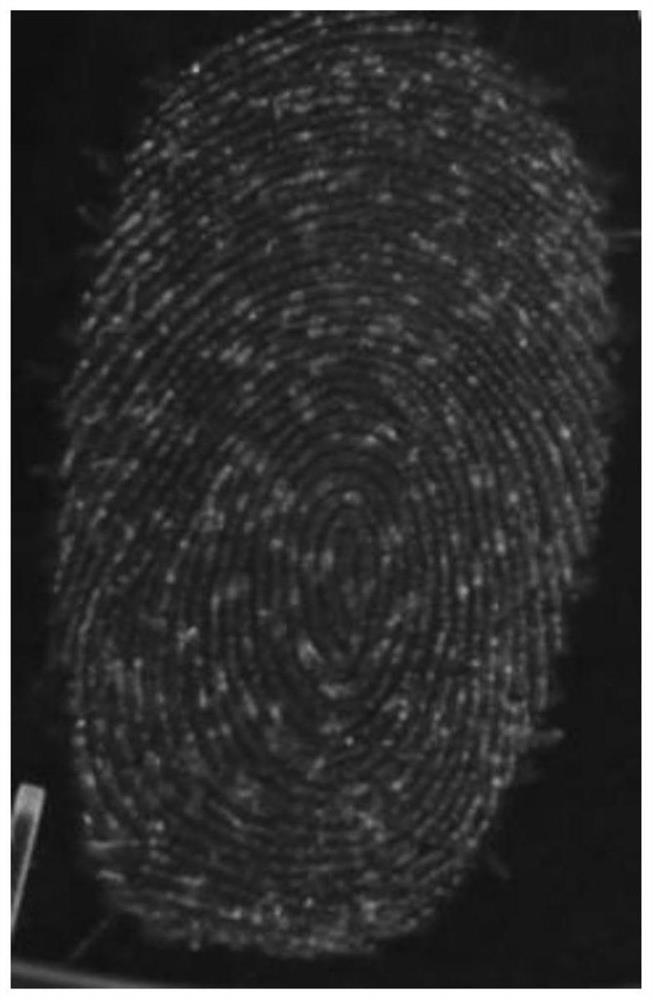
Application of a diarylethene compound in fluorescent color development of fingerprints
A diarylethene compound technology, applied in the application field of diarylene compounds in fingerprint fluorescent color development, to achieve the effects of increased polarity, high contrast, and not easy to aggregate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] A kind of compound, it is compound T1, the representative method of synthetic compound T1 is as follows:
[0168]
[0169] Compound 1 (200mg, 0.733mmol, 1eq.), compound 2 (159mg, 0.733mmol, 1eq.) and piperidine (2 drops) in EtOH (3mL) were reacted at 80°C for 16h. Concentrated under reduced pressure, the crude product was purified by flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0-15%) to obtain red solid compound T1 (220 mg, yield 64%). Mass Spectrum (ESI, m / z): 393.3 (M-Br) + .
[0170] Compound T1 is hydrophilic, soluble in water, and also has certain oil solubility, for example, it can be better dissolved in methanol, ethanol, DMF, DMSO, acetonitrile, tetrahydrofuran, dichloromethane and other oil-soluble solvents; insoluble in n-hexane , diethyl ether and ethyl acetate and other less polar solvents. Compound T1 has almost no fluorescence in aqueous solution, and emits strong orange-red fluorescence in 99% ethyl acetate / water system,...
Embodiment 2
[0180] A diarylethene compound T2, which has the following structural formula:
[0181]
[0182] The method of synthesizing this compound:
[0183] Compound 3 (363mg, 1.00mmol, 1eq.), compound 4 (262mg, 1.00mmol, 1eq.) and piperidine (3 drops) in EtOH (5mL) were reacted at 80°C for 16h. Concentrated under reduced pressure, the crude product was purified by flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0-18%) to obtain a red solid (271 mg, yield 48%). Mass Spectrum (ESI, m / z): 483.3 (M-Br) + .
[0184] The compound has amphiphilicity, has weak fluorescence in aqueous solution, emits strong orange-red fluorescence in 99% ethyl acetate / water system, and has remarkable aggregation-induced luminescence effect.
[0185] The compound fluoresces strongly against the words lipids, indicating that the probe is indeed able to identify lipids in sweat latent fingerprints.
Embodiment 3
[0187] A diarylethene compound T3, which has the following structural formula:
[0188]
[0189] The method of synthesizing this compound:
[0190] Compound 5 (546mg, 2.00mmol, 1eq.), compound 6 (432mg, 2.4mmol, 1eq.) and piperidine (6 drops) in MeCN (30mL) were reacted at 80°C for 16h. Concentrate under reduced pressure, the crude product is purified by flash preparative liquid chromatography (mobile phase gradient: DCM / MeOH=0~20%), and the intermediate product T3-1 that will be separated is dissolved in the mixed solution of 50mL ethanol / water (volume ratio is 9:1), add LiOHH 2 O (419 mg, 10 mmol), after stirring overnight at room temperature, 1M hydrochloric acid was added dropwise to adjust the Ph value to 2-3. The organic solvent was removed by rotary evaporation, extracted three times with dichloromethane, and the combined extract was concentrated and separated by flash liquid phase preparative chromatography (mobile phase gradient: DCM / MeOH=0~25%) to obtain a red s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com