Edasalonexent dosing regimen for treating muscular dystrophy
A technology for muscular dystrophy and drug delivery, which can be used in pharmaceutical formulations, drug combinations, drug delivery, etc., and can solve problems such as uncontrolled inflammation and pathology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0137] The present disclosure is further illustrated by the following examples, which should not be construed to limit the scope or spirit of the present disclosure to the specific procedures described herein. It should be understood that the examples are provided for the purpose of illustrating certain embodiments and are not intended thereby to limit the scope of the disclosure.
example 1
[0138] Example 1: Evaluation of the Pharmacokinetics of Ednaxen Dosing Regimen in C57BL / 6 Mice
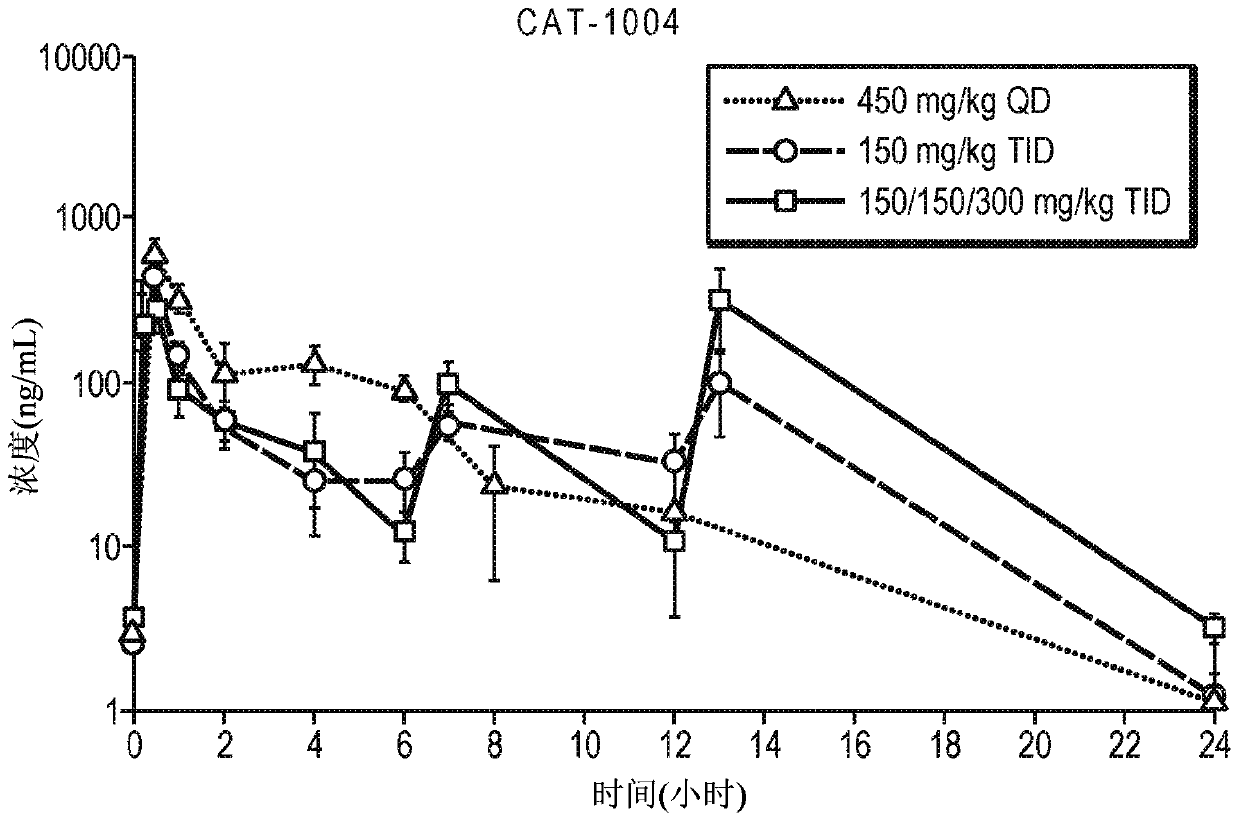
[0139] Ednaxen was administered to C57BL / 6 mice in the diet and / or by oral gavage. Pharmacokinetic (PK) modeling was used to identify the appropriate dosing amount and timing for the doses of 67 mg / kg / day and 100 mg / kg / day that have been tested in idenaxan human clinical trials. Specifically, mice were administered 1.5% idenaxen in the diet; 450 mg / kg / day orally delivered as a single daily dose; 450 mg / kg / day orally delivered as three equal doses; or as two 150 600 mg / kg / dose was delivered orally with a 300 mg / kg / dose and a 300 mg / kg / dose. For the dosing regimen of three daily doses, doses are delivered at 7:00am, noon and 5:00pm.
[0140] The dosing regimen for each group is depicted in Table 1. Each group contained 12 mice.
[0141] Table 1
[0142]
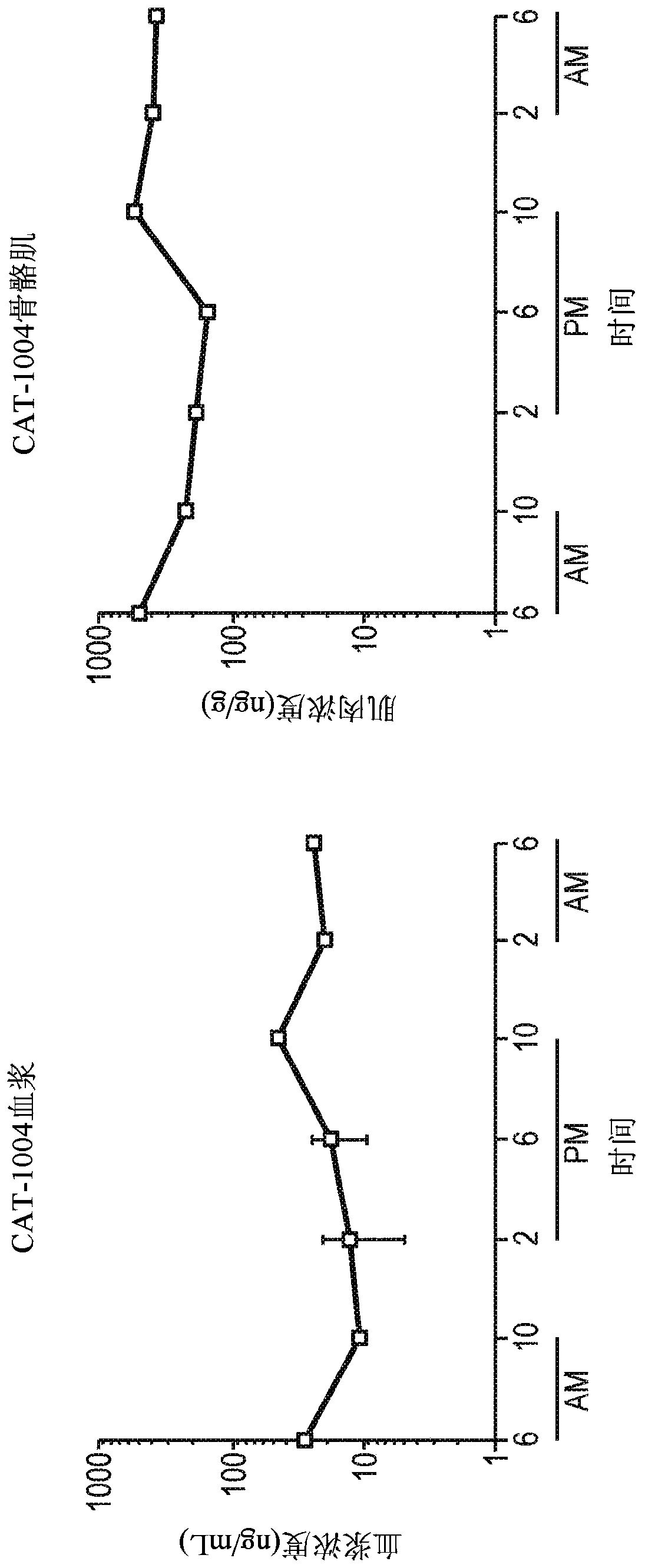
[0143] For animals administered idenaxen in the diet, idenaxen concentrations were measured by LC / MS / MS every 4 hours for ...
example 2
[0150] Example 2: Evaluation of idenaxen dosing regimen in mdx mice
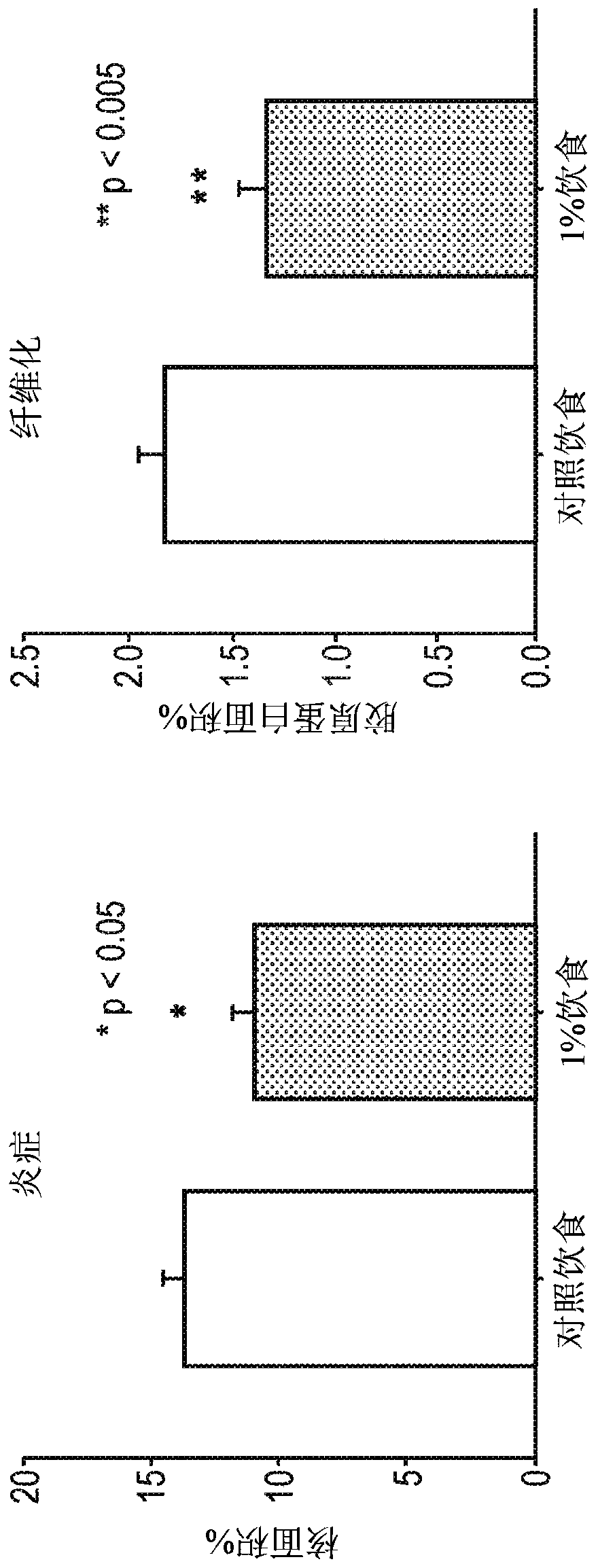
[0151]Young mdx mice were treated with different idenaxan dosing regimens for four weeks. The mdx mouse is a useful and well-accepted animal model for the study of Duchenne muscular dystrophy (DMD) (Mann et al. (2001) Proceedings of the National Academy of Sciences of the United States of America (PROC.NATL.ACAD.SCI.) 98(1):42-7). mdx mice lack expression of full-length dystrophin due to an inherited mutation within the dystrophin gene.
[0152] Ednaxen was administered to mice in the diet and / or by oral gavage. Specifically, ednaxen was administered to mice at 1% in their diet; 450 mg / kg / day was orally delivered in a single daily dose; 450 mg / kg / day was orally delivered in three equal doses; and / or 600 mg / kg / day was delivered orally in two 150 mg / kg / doses and one 300 mg / kg / dose. For the dosing regimen of three daily doses, doses are delivered at 7:00am, noon and 5:00pm.
[0153] The dosing regimen for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com