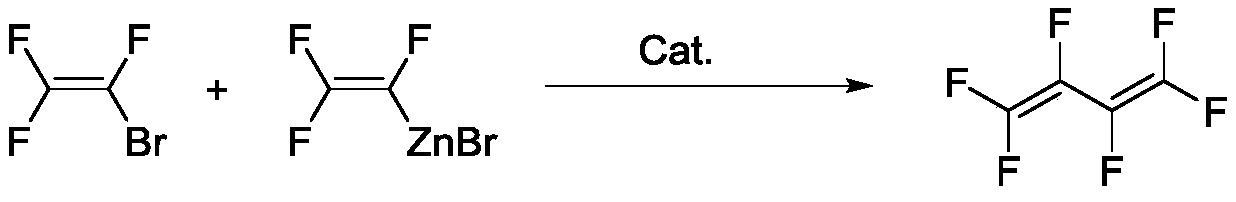
Method for preparing hexafluorobutadiene
A technology of hexafluorobutadiene and bromotrifluoroethylene, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of increased production cost, increased amount of three wastes, and unfavorable industrial application, etc., reaching The effect of reducing raw material cost, improving raw material utilization, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add palladium chloride 0.71g (4mmol) in the 500mL three-necked glass reactor that condensing reflux tube is housed, triphenylphosphine 4.2g (16mmol), then add the N of trifluorovinyl zinc bromide in this reaction bottle, N-dimethylformamide solution 380g (mass fraction 24%, 0.4mol), control internal temperature 20 ℃, slowly pass through 64.0g of trifluoroethylene bromide, the gas generated is collected by liquid nitrogen cold trap. After the passage of bromotrifluoroethylene, the reaction solution was kept warm for 3 hours, then heated up to 130° C., and all the gas was evaporated. A total of 54.5 g of the gas was collected and analyzed by chromatography. The results are shown in the following table:
[0038] Trifluoroethylene Bromotrifluoroethylene Hexafluorobutadiene other 2.77% 4.83% 91.80% 0.60%
[0039] Based on bromotrifluoroethylene, the reaction yield was 77.2%.
Embodiment 2
[0041] Add 0.90g (4mmol) of palladium acetate and 4.2g (16mmol) of triphenylphosphine in a 500mL three-necked glass reactor equipped with a condensing reflux tube, and then add N, N of trifluorovinyl zinc bromide to the reaction flask. - Dimethylformamide solution 380g (mass fraction 24%, 0.4mol), control internal temperature 20 ℃, slowly pass through 64.0g trifluoroethylene bromide, the gas generated is collected by liquid nitrogen cold trap. After the passage of bromotrifluoroethylene, the reaction solution was kept warm for 3 hours, then heated up to 130° C., and all the gas was evaporated. A total of 56.5 g of the gas was collected and analyzed by chromatography. The results are shown in the following table:
[0042] Trifluoroethylene Bromotrifluoroethylene Hexafluorobutadiene other 2.51% 10.30% 86.80% 0.39%
[0043] Based on bromotrifluoroethylene, the reaction yield is 75.7%.
Embodiment 3
[0045] Add palladium diacetylacetonate [Pd(acac) 2 ] 1.22g (4mmol), diphenylethoxyphosphine 3.68g (16mmol), then in this reaction bottle, add the N of trifluorovinyl zinc bromide, N-dimethylformamide solution 380g (mass fraction 24 %, 0.4mol), and the internal temperature was controlled at 20°C, and 64.0 g of bromotrifluoroethylene was slowly introduced, and the generated gas was collected with a liquid nitrogen cold trap. After the passage of bromotrifluoroethylene, the reaction solution was kept warm for 3 hours, then heated up to 130° C., and all the gas was evaporated. A total of 53.9 g of the gas was collected and analyzed by color chromatography, and the results were as follows;
[0046] Trifluoroethylene Bromotrifluoroethylene Hexafluorobutadiene other 3.51% 25.58% 70.21% 0.70%
[0047] Based on bromotrifluoroethylene, the reaction yield was 58.4%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap