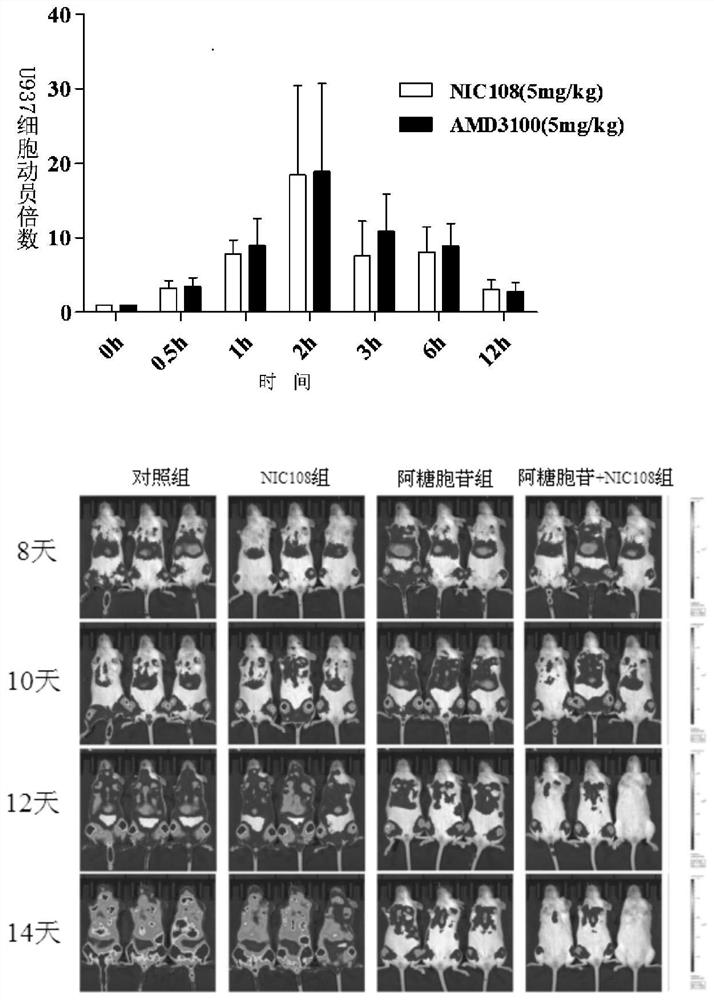
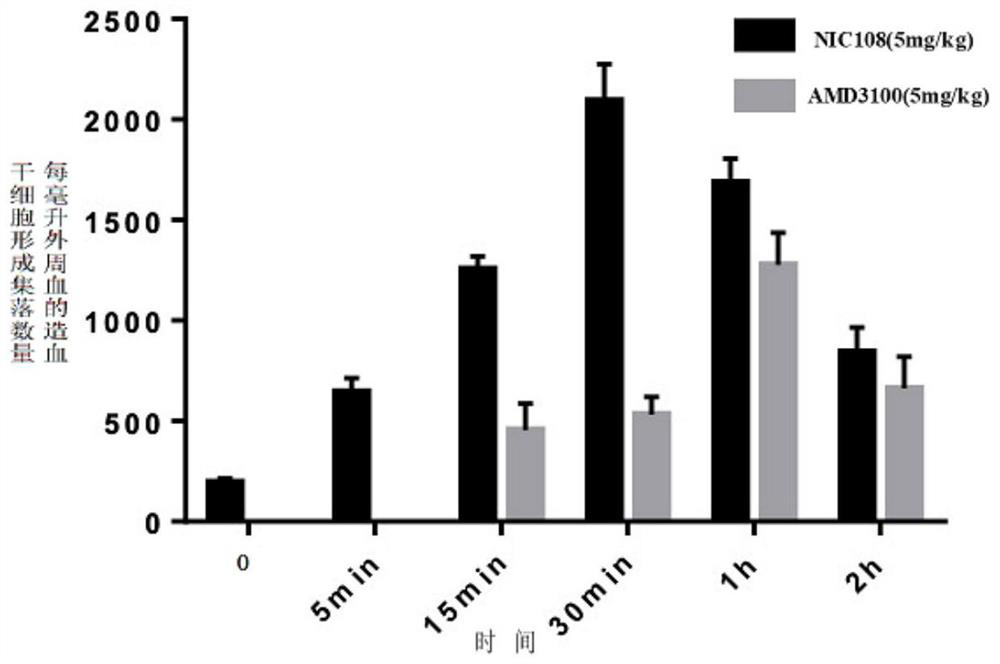
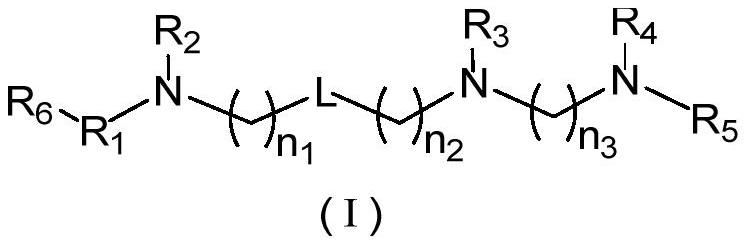
cxcr4 inhibitors and their applications
A technology selected from and compound, applied in the field of medicine, can solve the problems of less drugs and unable to meet the needs of patients, and achieve the effects of strong inhibitory activity, high biological activity and availability, and long drug effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137]
[0138] Synthesis of Intermediate 71-2:
[0139] Compound 71-1 (500 mg, 2.92 mmol) was dissolved in THF (30 mL) and H 2 O 30mL, add sodium bicarbonate (294mg, 3.50mmol), di-tert-butyl dicarbonate (764mg, 3.50mmol), and stir at room temperature. TLC followed the reaction until the raw material methyl tranexamic acid disappeared, the reaction was stopped, extracted three times with saturated sodium bicarbonate and DCM, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, and purified by column (PE:EA=3:1) , to obtain intermediate 6-2 (751 mg).
[0140] Synthesis of intermediate 71-2:
[0141] Dissolve 71-1 (750mg, 2.77mmol) in THF (30mL), cool down to 0°C and stir, slowly add LiAlH4 (211mg, 5.54mmol), stir, and react at room temperature. TLC followed the reaction until the starting material 83-1 disappeared, then stopped the reaction, extracted three times with saturated saline and EA, combined the organic phases, dried over anhydrou...
Embodiment 2
[0167]
[0168] Synthesis of Intermediate 96-2:
[0169] Dissolve compound 96-1 (400mg, 0.84mmol) in 10Ml THF, add 2,4-dichloropyrimidine (125mg, 0.84mmol) and DIEA (129mg, 1.00mmol) successively, stir at room temperature, follow the reaction by TLC, to the starting material 96-1 disappeared, stopped the reaction, extracted three times with saturated saline and DCM, combined the organic phases, dried over anhydrous sodium sulfate, concentrated, and purified by column (DCM:CH 3 OH=30:1), the final product 96-2 (392 mg) was obtained as a yellow solid.
[0170] Synthesis of Intermediate 96-3:
[0171] Compound 96-2 (390mg, 0.66mmol) was dissolved in 20Ml 1,4-dioxane, and 2-aminomethylpyridine (71mg, 0.66mmol), cesium carbonate (323mg, 0.99mmol) and [1 , 1'-bis(diphenylphosphino)ferrocene]palladium dichloride (48mg, 0.066mmol), replaced by N2, heated to 100°C for reaction. TLC followed the reaction until the raw material 96-2 disappeared, stopped the reaction, extracted with...
Embodiment 3
[0179]
[0180] Synthesis of intermediate 17-2:
[0181] Dissolve N-Fmoc-L-alanine (295mg, 0.95mmol) in THF (20Ml), stir, add HATU (431mg, 1.14mmol), HOBT (153mg, 1.14mmol), DIEA (244mg, 1.89mmol) ) and compound 17-1 (528mg, 0.95mmol), stirred at room temperature, TLC followed the reaction until the disappearance of raw material 17-1, stopped the reaction, extracted three times with saturated sodium bicarbonate and DCM, combined the organic phases, dried over anhydrous sodium sulfate, concentrated After column purification (PE:EA=3:1), intermediate 17-2 (679 mg) was obtained.
[0182] Synthesis of Intermediate 17-3:
[0183] Compound 17-2 (650 mg) was dissolved in 1M PIP / THF solution and stirred at room temperature. TLC followed the reaction until the disappearance of raw material 17-2, stopped the reaction, concentrated and purified by column (DCM:CH 3 OH=10:1), to obtain intermediate 17-3 (451 mg).
[0184] Synthesis of intermediate 17-4:
[0185] Compound 17-3 (450m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap