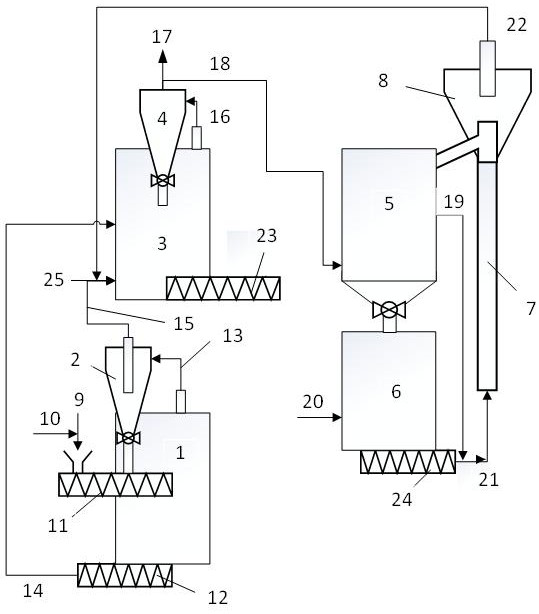
A biomass microwave gasification utilization method and system
A technology of biomass and biomass raw materials, applied in gasification process, manufacture of combustible gas, petroleum industry, etc., can solve problems such as poor quality of synthesis gas and hydrogen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] According to the molar ratio of ferric nitrate, nickel nitrate and titanium sulfate 1:1:0.34, weigh 0.625 mol of ferric nitrate, 0.625 mol of nickel nitrate and 0.2125 mol of titanium sulfate to make 1L solutions respectively. In a reaction vessel with 50g of magnesium oxide, under stirring conditions, 3L of urea solution (2mol / L) was mixed dropwise and stirred for 4 hours, and then the ferric nitrate and nickel nitrate solutions were added dropwise in parallel was added, and the co-precipitation reaction was continued for 4 hours. During this process, a precipitate gradually formed. After the reaction was complete, it was centrifuged, and the precipitate was filtered and washed with deionized water to neutrality. The precipitate was dried at 60°C for 12 hours, then burned at 950°C for 8 hours, and obtained after natural cooling. Oxygen carrier A (31wt%Fe 2 o 3 , 29wt%NiO, 10wt%TiO 2 , 30wt%MgO).
Embodiment 2
[0061] According to the molar ratio of ferric nitrate, copper nitrate and zirconium sulfate 1:0.18:0.09, weigh 0.625 mol of ferric nitrate, 0.1125 mol of copper nitrate and 0.05625 mol of zirconium sulfate to make 1L solutions respectively. Contains 20g α-Al 2 o 3In the reaction vessel, under stirring conditions, 3L urea solution (2mol / L) was mixed dropwise and stirred for 4h, and then ferric nitrate and copper nitrate solutions were added dropwise in parallel, and the co-production was continued. The precipitation reaction was 4 hours. During this process, a precipitate gradually formed. After the reaction was complete, it was centrifuged, and the precipitate was filtered and washed with deionized water to neutrality. The precipitate was dried at 60°C for 12 hours, then burned at 950°C for 8 hours, and obtained after natural cooling. Oxygen carrier B (58.2wt%Fe 2 o 3 , 10.5wt%CuO, 8.0wt%ZrO 2 , 23.3wt%Al 2 o 3 ).
Embodiment 3
[0063] According to the molar ratio of ferric nitrate, copper nitrate and titanium sulfate 1:0.4:0.2, weigh 0.625 mol of ferric nitrate, 0.25 mol of copper nitrate and 0.1125 mol of titanium sulfate to make 1L solutions respectively. Contains 20g α-Al 2 o 3 In the reaction vessel, under stirring conditions, 3L urea solution (2mol / L) was mixed dropwise and stirred for 4h, and then ferric nitrate and copper nitrate solutions were added dropwise in parallel, and the co-production was continued. The precipitation reaction was 4 hours. During this process, a precipitate gradually formed. After the reaction was complete, it was centrifuged, and the precipitate was filtered and washed with deionized water to neutrality. The precipitate was dried at 60°C for 12 hours, then burned at 950°C for 8 hours, and obtained after natural cooling. Oxygen carrier C (50.5wt%Fe 2 o 3 , 20.2wt%CuO, 9.1wt%TiO 2 , 20.2wt%Al 2 o 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com