Ruco alloy catalyst and its preparation method and application for ammonia synthesis
An alloy catalyst, ammonia synthesis technology, applied in chemical instruments and methods, physical/chemical process catalysts, ammonia preparation/separation, etc. The effect of high utilization and high atomic dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] According to the present invention, there is also provided a method for producing an ammonia-based RUCO alloy catalyst, including the following steps: 1) Take the melamine, the Ru precursor, and the CO precursor into the dimethyl sulfoxide (DMSO) solution to obtain a mixture. The mixed solution is ultrasonic; 2) Take the mixture of triocyanoate into the DMSO solution, ultrasonic; 3), slowly pour the mixed liquid obtained in step 2 into step 1), stir, stir filter, and Washing with water and ethanol solution in sequentially, the Ruco alloy catalyst was obtained under an inert atmosphere.
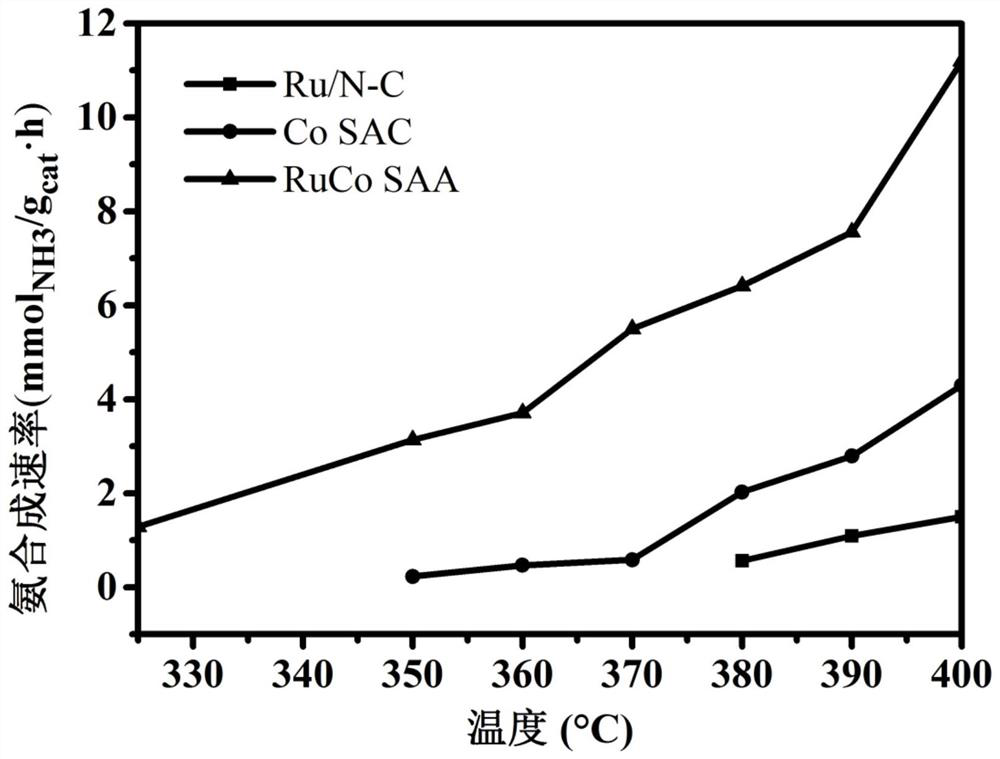
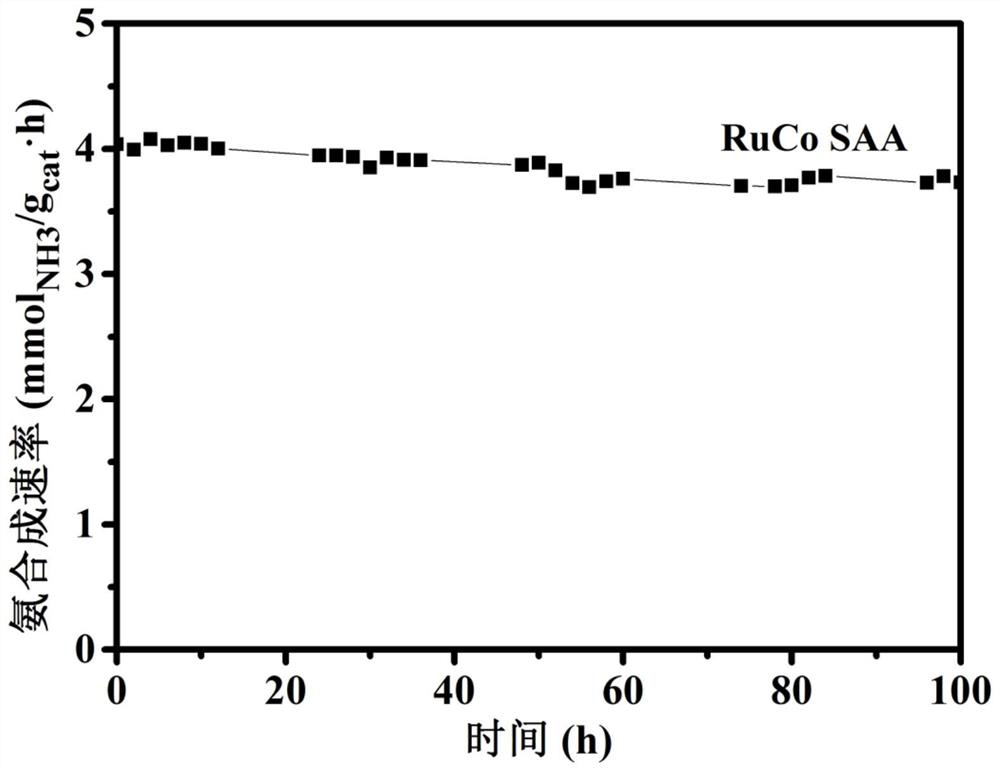
[0027]The present invention is a highly dispersed synthetic RuCo monoatomic alloy catalyst which can break the current limiting relationship ammonia synthesis catalyst during the reaction. It is a prior art N 2 Dissociative adsorption on the metal surface, and then gradually hydrogenated to produce ammonia, the present invention is N 2 Ru is adsorbed on the active site, and then hydrogenati...
Embodiment 1
[0037] 1), respectively, 0.5g of melamine (C 3 Hide 6 N 6 ), Cobalt phthalocyanine 0.201 g, 0.35 mL solution of ruthenium nitrosyl nitrate dissolved in 40mL DMSO solution, and the mixed solution was sonicated for 10 min.
[0038] 2) 0.51g cyanuric acid (C 3 Hide 3 N 3 O 3 ) Was dissolved in 10mL of DMSO, sonicated 10min.
[0039] 3) The step 2) the resulting solution was slowly poured Step 1) was obtained at a rate of 600r / min was stirred 10min, filtered off with suction, and washed with 150mL deionized water and 100mL ethanol solution, after the samples were washed at 60 ℃ for dried for 12 hours.
[0040] 4) The sample was dried in an Ar atmosphere at a heating rate of 1 ℃ / min raised to 600 ℃, and holding 8h, to give a nitrogen-doped carbon support RuCo monoatomic alloy catalyst, SAA RuCo labeled.
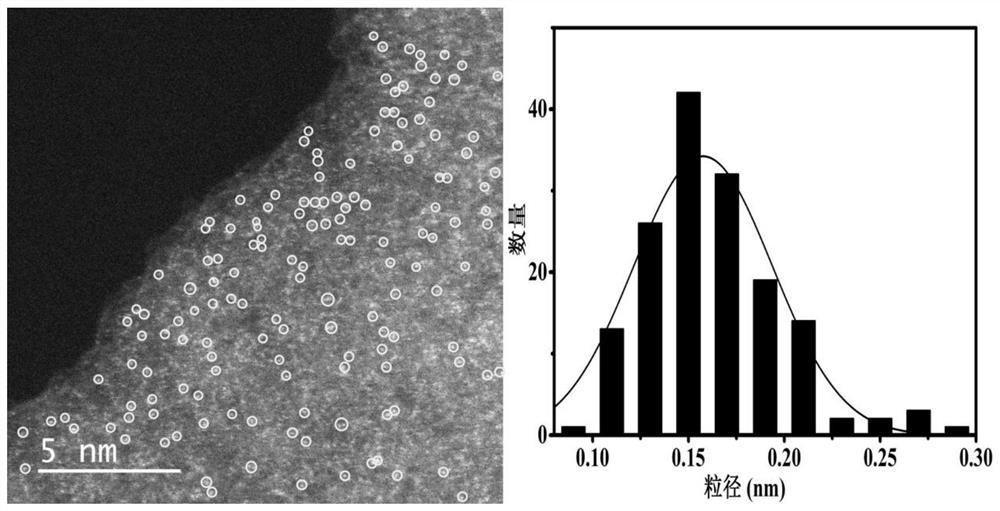
[0041] Ru using ICP-AES (inductively coupled plasma atomic emission spectroscopy) as measured in Example embodiments of the load is 0.34%, Co 6.6%. Using N 2 Adsorption-desorption...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com