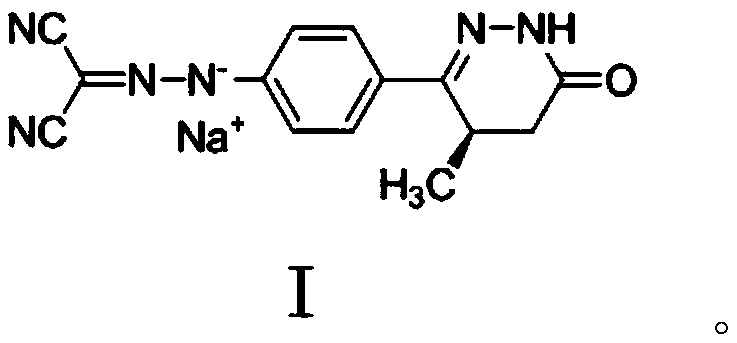
Levosimendan sodium crystal form and preparation method thereof
A technology for sodium and sodium crystals of levosimendan, which is applied in the field of levosimendan sodium crystal form and its preparation, can solve the problems of insolubility and irritation of levosimendan, achieve simple production process and operation, save energy, Effects of avoiding irritation and hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of levosimendan sodium crystal form A
[0059] Add 2.9g of sodium hydroxide and 120ml of purified water into a 250ml flask, stir and dissolve at room temperature. Add 20 g of levosimendan and stir to dissolve levosimendan. Stir and crystallize at 5-10°C for 2 hours. Filter and wash the filter cake with 30ml of absolute ethanol. Dry under reduced pressure at 60°C to obtain 17.8 g of crystalline form A levosimendan sodium, with a weight yield of 89.0%.
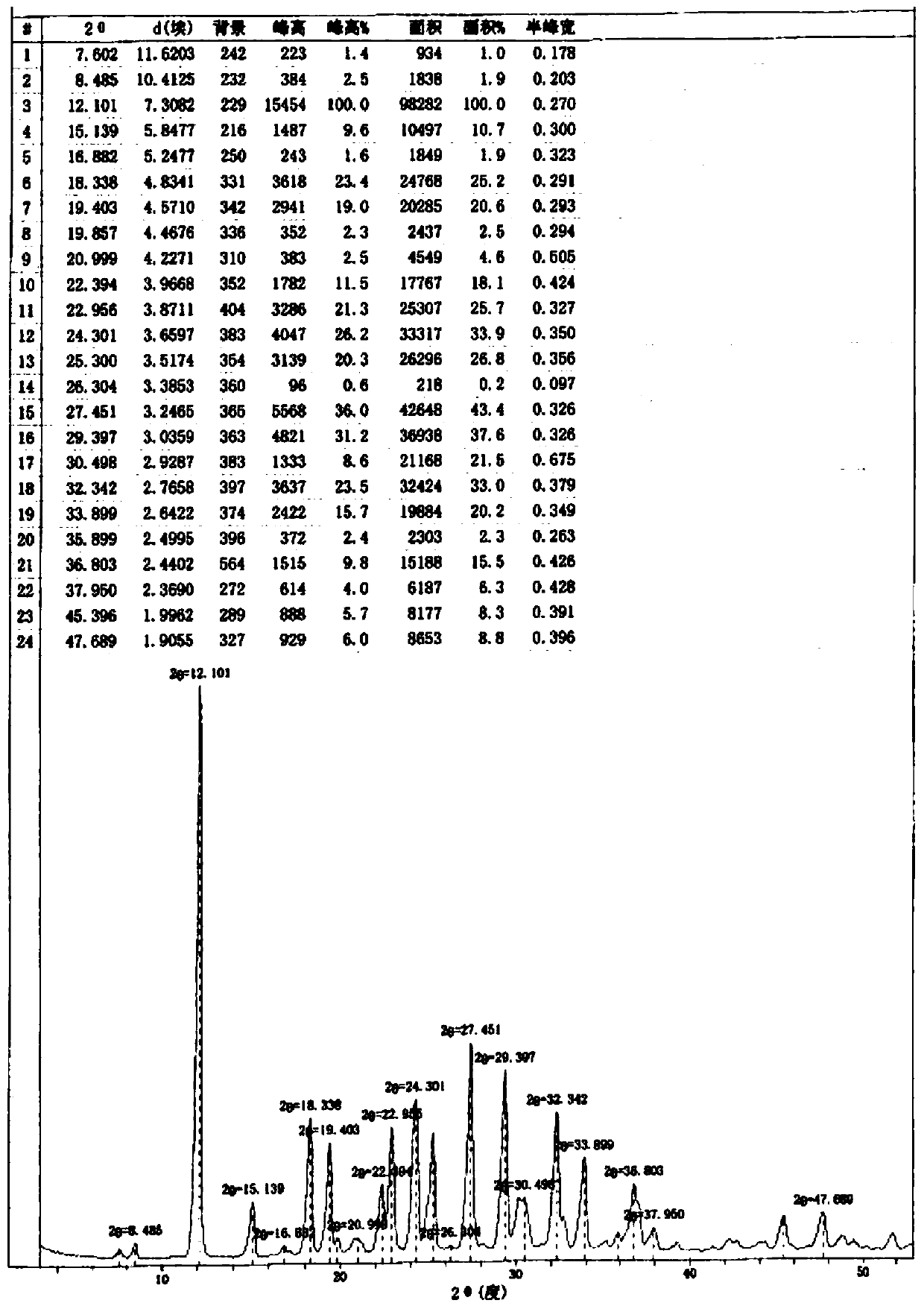
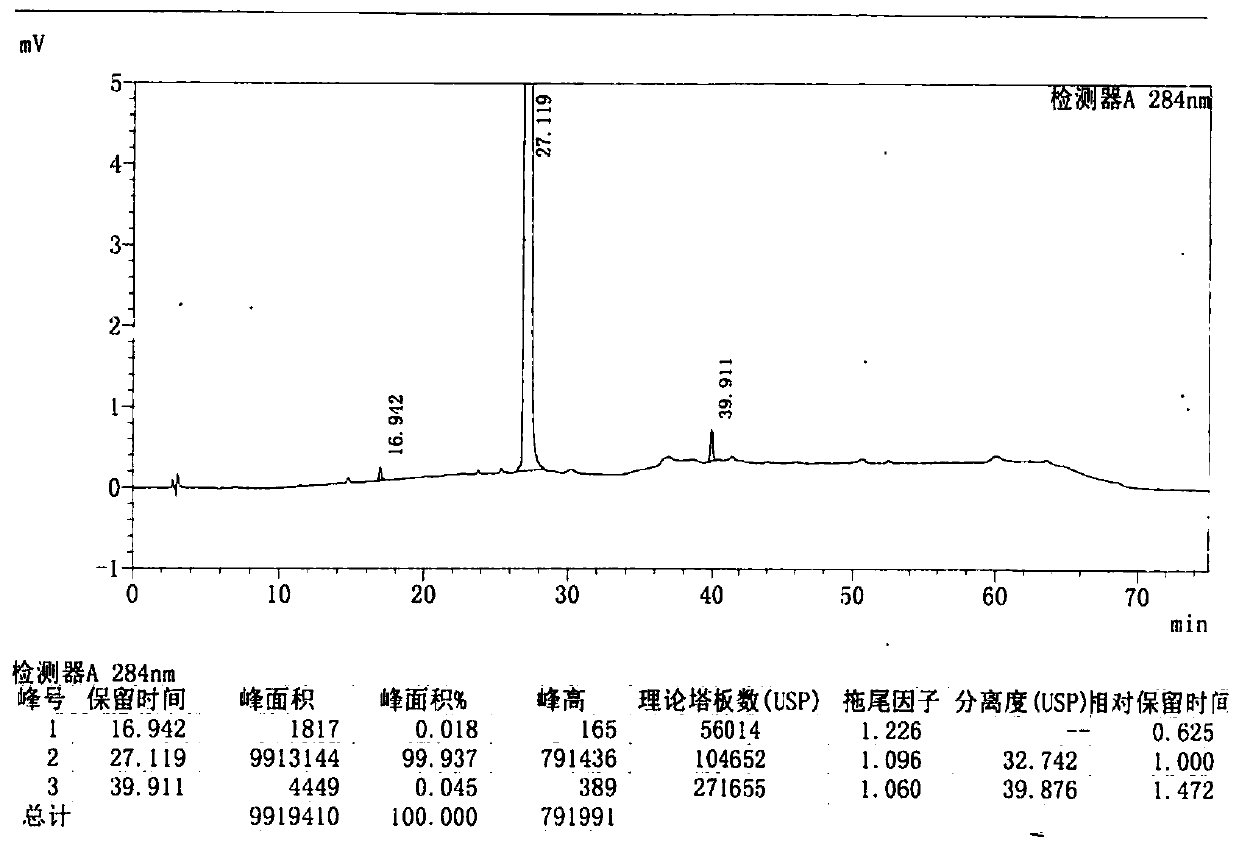
[0060] After testing, its X-ray powder diffraction pattern is consistent with figure 1 Basically the same, HPLC purity and figure 2 Basically the same.
[0061] figure 1 Among them, crystalline form A levosimendan sodium has characteristic peaks at 2θ of 12.101°, 15.139°, 18.338°, 19.403°, 22.956°, 24.301°, 27.451°, 29.397°, 32.342°, 33.899°.
Embodiment 2
[0062] Embodiment 2: Preparation of levosimendan sodium crystal form A
[0063] Add 2.8g of sodium hydroxide and 200ml of absolute ethanol into a 500ml flask, and heat to about 40°C to dissolve the sodium hydroxide. Add 20g of levosimendan, stir into salt. Stir and crystallize at 10-15°C for 4 hours. Filter and wash the filter cake with 30ml of absolute ethanol. Dry under reduced pressure at 40°C to obtain 16.7 g of crystalline form A levosimendan sodium. The weight yield is 83.5%.
[0064] After testing, its X-ray powder diffraction pattern is consistent with figure 1 Basically the same, HPLC purity and figure 2 Basically the same.
Embodiment 3
[0065] Embodiment 3: Preparation of levosimendan sodium crystal form A
[0066] Add 3.9 g of sodium ethoxide and 150 ml of methanol into a 500 ml flask, and heat to about 40° C. to dissolve the sodium ethoxide. Add 20g of levosimendan, stir into salt. Stir and crystallize at 5-10°C for 5 hours. Filter and wash the filter cake with 20ml of methanol. Dry under reduced pressure at 40°C to obtain 15.0 g of crystalline form A levosimendan sodium, with a weight yield of 75.0%.
[0067] After testing, its X-ray powder diffraction pattern is consistent with figure 1 Basically the same, HPLC purity and figure 2 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com