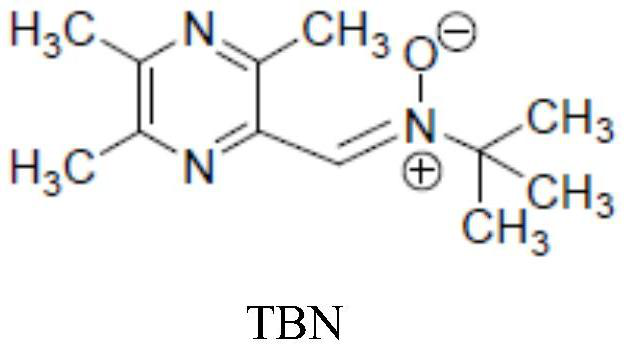
Pharmaceutical composition containing TBN or salt or hydrate of TBN and preparation method of pharmaceutical composition
A composition and hydrate technology, which is applied in the direction of drug combination, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of high toxicity and side effects, no specific drugs, poor curative effect, etc., and achieve improved bioavailability, Effect of improving stability and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Pass the TBN crude drug, mannitol, pregelatinized starch, crospovidone, sodium bicarbonate and magnesium stearate through a 40-mesh sieve, and set aside. Weigh 100g TBN raw material, 70g mannitol, 70g pregelatinized starch, 7.5g povidone K30, 5g crospovidone, 10g sodium bicarbonate, mix in a laboratory hopper mixer for 30min, then add 1g stearic acid Magnesium, mixed together for 1min, and directly compressed to obtain 1000 TBN tablet cores with a specification of 100mg and an average tablet weight of 0.2635g.
Embodiment 2
[0057] Pass the TBN crude drug, mannitol, pregelatinized starch, crospovidone, sodium bicarbonate, and magnesium stearate through a 40-mesh sieve, and set aside. Weigh 100g TBN raw material, 70g mannitol, 70g pregelatinized starch, 7.5g povidone K30, 5g crospovidone, 20g sodium bicarbonate, mix in a laboratory hopper mixer for 30min, then add 1g stearic acid Magnesium, mixed together for 1 min, and directly compressed to obtain 1000 TBN core tablets with a specification of 100 mg and an average tablet weight of 0.2735 g.
Embodiment 3
[0059] Pass the TBN crude drug, mannitol, pregelatinized starch, crospovidone, magnesium oxide, and magnesium stearate through a 40-mesh sieve, and set aside. Weigh 100g TBN API, 70g mannitol, 70g pregelatinized starch, 7.5g povidone K30, 5g crospovidone, 20g magnesium oxide, mix in a laboratory hopper mixer for 30min, then add 1g magnesium stearate , mixed together for 1 min, and directly compressed to obtain 1000 TBN tablet cores with a specification of 100 mg and an average tablet weight of 0.2735 g.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap