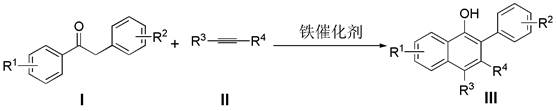
A kind of synthetic method of β-aryl-α-naphthol compound
A synthesis method and compound technology, applied in the field of synthesis of β-aryl-α-naphthol compounds, can solve the problems of rare metal resources, low price of precious metal resources, low atom economy, lengthy synthesis steps, etc., and achieve a wide range of substrates , High atomic utilization rate, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add deoxybenzoin compound Ia (1.0 mmol) and ferric oxide (1.0 mmol) into a 10 mL round bottom flask, seal with a rubber stopper and evacuate, then use an argon balloon to replace the gas in the reaction bottle, so that the bottle is filled with argon gas. Finally, the solvent xylene (3.0 mL) and alkyne compound IIa (2.0 mmol) were added to the reaction flask under argon atmosphere, and the reaction mixture was stirred at 135 °C for 24 h (the reaction time and temperature were determined by different bases Material decision), thin-layer chromatographic plate detection reaction, until the raw material reaction is complete. column chromatography β -Aryl- α - Naphthol compound IIIa (82% yield).
[0018]
[0019] White solid; Melting point: 103 – 104°C; 1 H NMR (400 MHz, DMSO) δ 9.44 (s, 1H), 8.42 (d, J = 8.3 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 7.68 (d, J = 7.5 Hz, 2H),7.56 – 7.56 (m, 1H), 7.51 – 7.39 (m, 8H), 7.37 – 7.31 (m, 2H); 13 C NMR (100MHz, DMSO) δ 148.6...
Embodiment 2
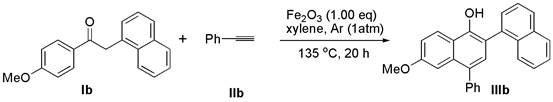
[0021] Add deoxybenzoin compound Ib (1.0 mmol) and ferric oxide (1.0 mmol) into a 10 mL round bottom flask, seal with a rubber stopper and evacuate, then use an argon balloon to replace the gas in the reaction bottle, so that the bottle is filled with argon gas. Finally, the solvent xylene (3.0 mL) and alkyne compound IIb (2.0 mmol) were added to the reaction flask under argon atmosphere, and the reaction mixture was stirred at 135 °C for 24 h (the reaction time and temperature were determined by different bases Material decision), thin-layer chromatographic plate detection reaction, until the raw material reaction is complete. column chromatography β -Aryl- α - Naphthols IIIb (72% yield).
[0022]
[0023] 6'-methoxy-4'-phenyl-[1,2'-binaphthalen]-1'-ol (IIIb): white solid; melting point: 83 – 84 o C; 1 H NMR (400 MHz, CDCl 3 ) δ 8.31 (d, J = 9.2 Hz, 1H), 7.94 (d, J = 6.4 Hz, 2H), 7.78 (d, J = 8.3 Hz, 1H), 7.62 – 7.50 (m, 5H), 7.47 – 7.44 (m,3H), 7.40 – 7.30 (m, ...
Embodiment 3
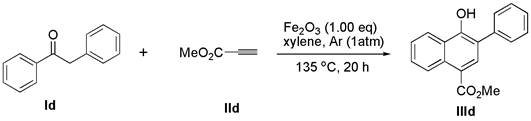
[0025] Add deoxybenzoin compound Ic (1.0 mmol) and ferric oxide (1.0 mmol) into a 10 mL round bottom flask, seal with a rubber stopper and evacuate, then use an argon balloon to replace the gas in the reaction bottle, so that the bottle is filled with argon gas. Finally, the solvent xylene (3.0 mL) and alkyne compound IIc (2.0 mmol) were added to the reaction flask under argon atmosphere, and the reaction mixture was stirred at 135 °C for 24 h (the reaction time and temperature were determined by different bases Material decision), thin-layer chromatographic plate detection reaction, until the raw material reaction is complete. column chromatography β -Aryl- α - Naphthol compound IIIc (77% yield).
[0026]
[0027] White solid; melting point: 117 – 118 o C; 1 H NMR (400 MHz, DMSO) δ 9.33 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.67 (d, J = 7.2 Hz, 2H),7.58 – 7.39 (m, 6H), 7.35 (t, J = 7.4 Hz, 1H), 7.28 (s, 1H), 7.06 (d, J = 8.6Hz, 2H), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com