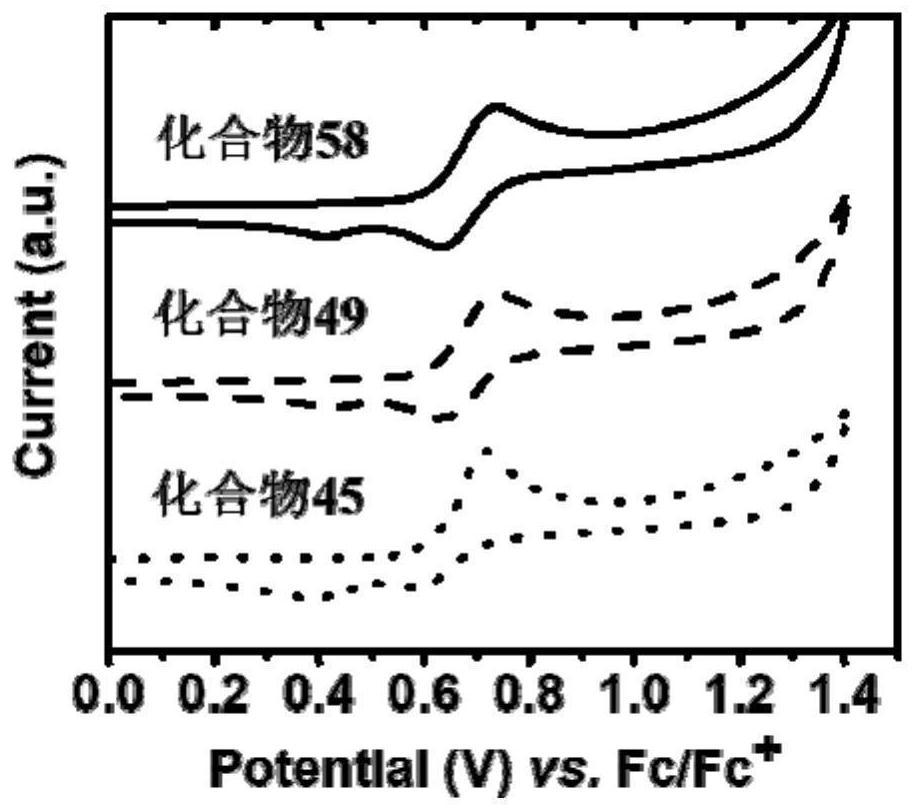
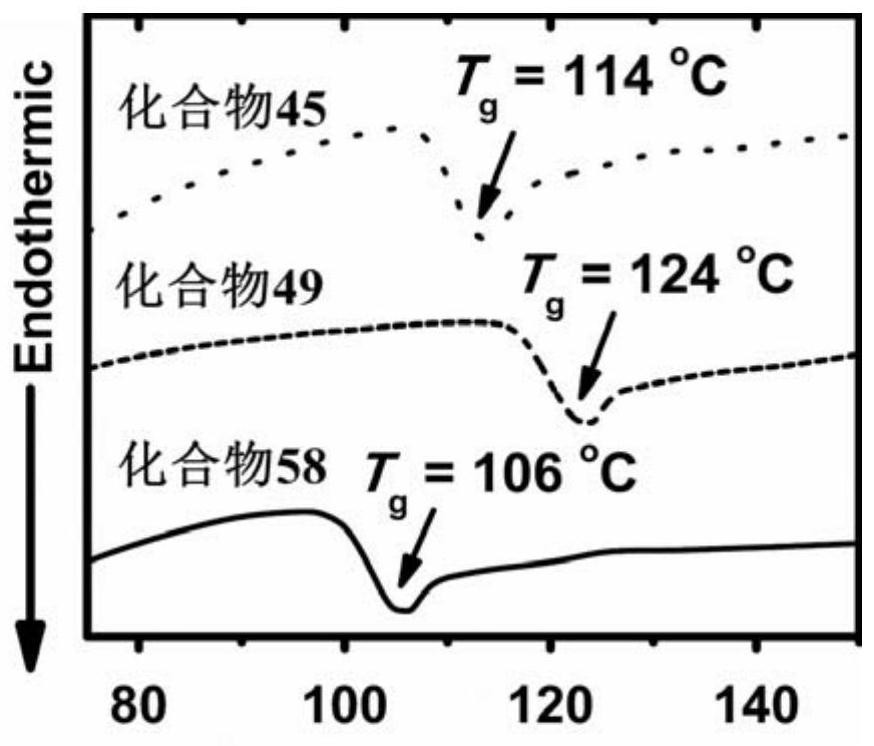
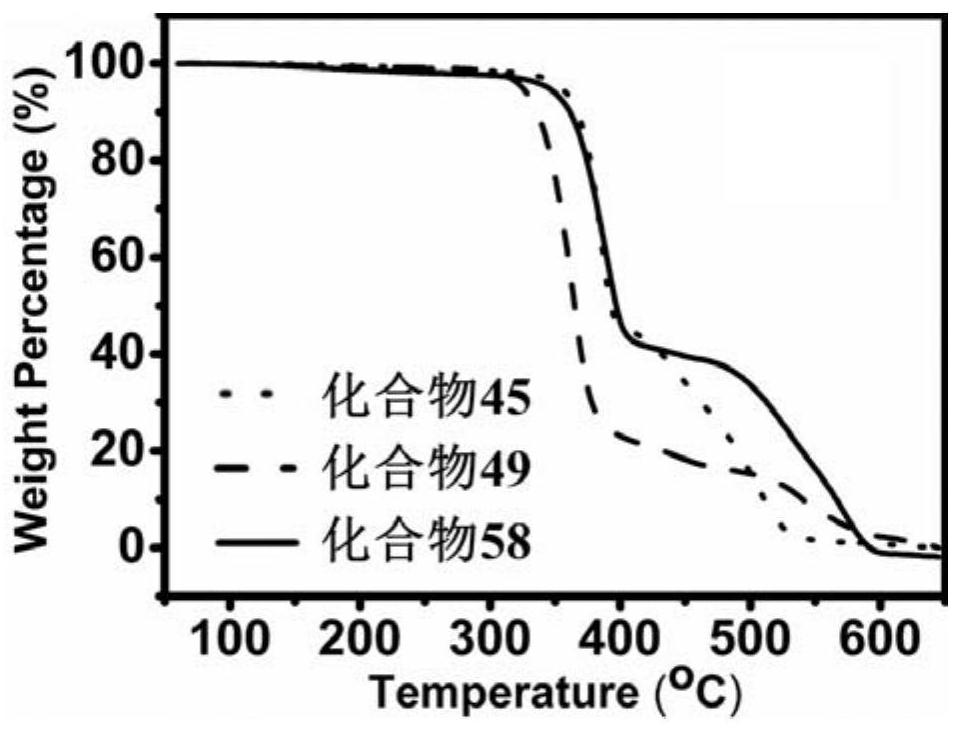
A thermally activated delayed fluorescent material and electroluminescent device
An electroluminescent device, thermal activation delay technology, applied in luminescent materials, electro-solid devices, electrical components, etc., can solve the problem of low luminous efficiency of thermally activated delayed fluorescent materials, and achieve effective regulation of luminescence performance, enhanced interaction, Effects of Nonradiative Transition Suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of compound 11 comprises the following steps:
[0038] 1), the preparation reaction formula of intermediate 11-1 is as follows:
[0039]
[0040] Prepare a 25mL dry round-bottomed flask, add 4-bromodiphenyl sulfoxide (1.12g, 4.0mmol), sodium azide (0.31g, 4.8mmol) and 8mL chloroform at a temperature of 0 °C, add dropwise 1.60 mL of concentrated sulfuric acid was added, and the mixture was heated at 45 °C and stirred for 12 h. After the 4-bromodiphenyl sulfoxide was completely reacted, it was cooled to room temperature, the reaction was quenched with water, the organic layer was extracted with chloroform, washed with salt, the organic layer was dried with anhydrous sodium sulfate, filtered and concentrated, and the crude product was passed through the column layer Further purification by analytical column chromatography gave a colorless solid, intermediate 11-1 (0.83 g, 2.8 mmol), with HPLC purity of 99.3% and yield of 70%. MS(MALDI-TOF): m / z ...
Embodiment 2
[0048] The preparation method of compound 15 comprises the following steps:
[0049] 1), the preparation reaction formula of intermediate 15-1 is as follows:
[0050]
[0051] In a 50 mL round-bottomed flask was added 4-bromodiphenyl sulfoxide (1.13 g, 4.0 mmol, 1.0 equiv.), PhI(OAc) 2 (3.87 g, 12.0 mmol, 3.0 equiv.), ammonium carbamate (1.25 g, 16.0 mmol, 4.0 equiv.) and methanol (8.0 mL), stirred openly at room temperature for 30 min and removed the solvent by rotary evaporation. The crude product was subjected to column chromatography Purification and drying in vacuo gave a colorless solid, Intermediate 15-1 (0.83 g, 2.80 mmol), HPLC purity 99.2%, yield 70%. MS(MALDI-TOF): m / z 294.9[M + ].
[0052] 2), the preparation reaction formula of intermediate 15-2 is as follows:
[0053]
[0054] Intermediate 15-1 (0.75g, 3.0mmol), triethylamine (0.68mL, 0.83g, 5.2mmol) and 50mL of dichloromethane were added to a 150mL single-neck round bottom flask, dissolved, cooled in a...
Embodiment 3
[0059] The preparation method of compound 45 comprises the following steps:
[0060] 1), the preparation reaction formula of intermediate 45-1 is as follows:
[0061]
[0062]2-Bromodibenzothiophene (2.62g, 10.0 mmol), iodobenzenediacetic acid (16.10g, 50.0mmol, 5equiv.), ammonium carbonate (2.88g, 30.0mmol, 3equiv.) were successively added to a 250mL single-necked round bottom flask. ) and 80 mL of methanol, the above mixture was stirred at room temperature for 48 hours. The solvent was removed by rotary evaporation, purified by column chromatography, and dried in vacuo to obtain 2.40 g (8.20 mmol) of a colorless solid with HPLC purity of 99.5% and yield of 82%. MS(MALDI-TOF): m / z 293.0[M + ].
[0063] 2), the preparation reaction formula of intermediate 45-2 is as follows:
[0064]
[0065] Intermediate 45-1 (0.75g, 2.6mmol), dissolved in 50mL dichloromethane, diphenylphosphine chloride (1.21g, 0.98mL, 5.2mmol) and pyridine (0.41mL) were added to a 150mL single-nec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com