Aza aromatic ring amide derivatives for use in treatment of cancer
A heterocyclic and heteroaryl technology, applied in the field of azaaromatic cyclic amides, can solve problems such as the lack of effective drugs targeting HER4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
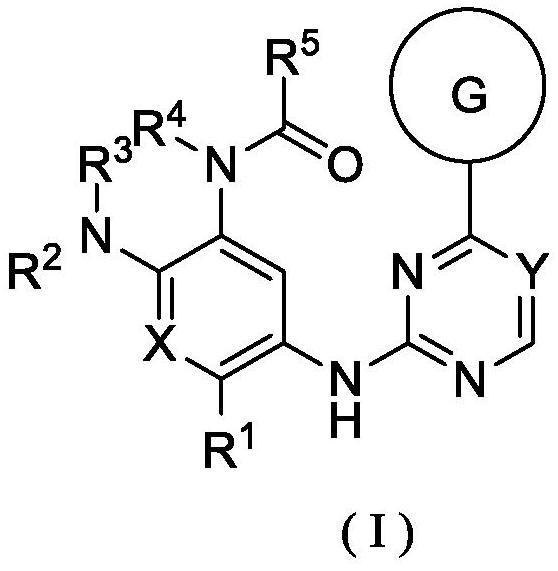
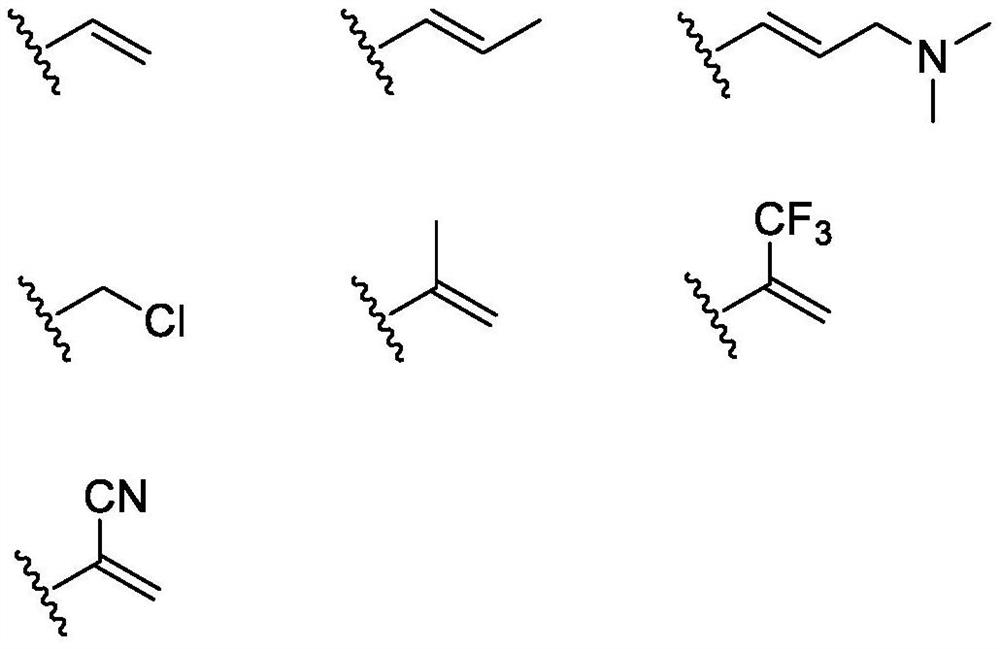
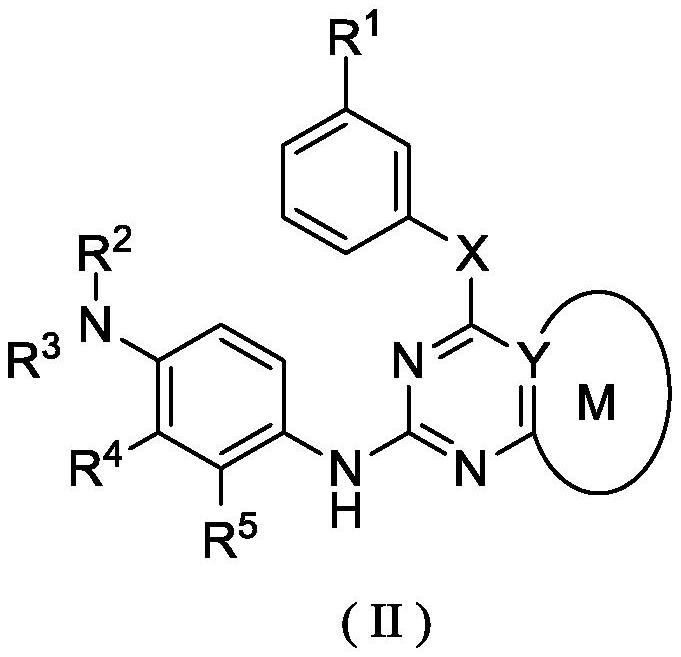
[0272] According to the compound of formula (I) described in the first aspect of the present invention, its chemical synthesis and preparation can refer to articles published by Zeng, QB, etc. in Journal of Medicinal Chemistry, 2015, 58, 8200-8215, and patent WO2018 / 210246 (A1 ), CN105461695A, CN201410365911A, CN201610126987A, CN109761960 and CN106928200A, which are selected from the following compounds:
[0273] N-(2-((2-(dimethylamino)ethyl(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl )-1,3,5-triazin-2-yl)amino)phenyl)acrylamide (compound 1);
[0274]
[0275] N-(5-((4-(3,3-Dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridin-1-yl)-1,3,5-tri (oxazin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide (compound 2);
[0276]
[0277] N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(3-methyl-1H-pyrrolo[3, 2-c]pyridin-1-yl)-1,3,5-triazin-2-yl)amino)phenyl)acrylamide (compound 3);
[0278]
[0279] N-(2-((2-(dimethylamino)e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com