Synthetic method and application of 4-oxo-trans-2-hexenal analogues
A synthesis method and hexenal technology are applied in the field of synthesizing sex pheromone analogs, which can solve the problems of short duration, fast field release, generation of insect resistance, etc., and achieve long duration and strong specificity. , strong anti-stress effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
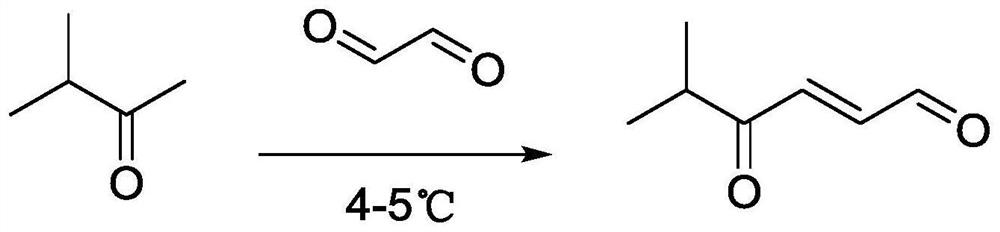
[0023] Example 1 The preparation method of 5-methyl-4-oxo-trans-2-hexenal
[0024] The synthetic route of the compound 5-methyl-4-oxo-2-hexenal in the present invention is as follows figure 2 shown.
[0025] In a 100mL flask, dissolve 1g (11.61mmol) of methyl isopropyl ketone in 30mL at a concentration of 0.08mol·L -1 In the sodium hydroxide solution, 0.85g (14.5mmol) of glyoxal solution was gradually added dropwise. The reaction process was monitored by TLC. After the raw materials were no longer reduced, the reaction was complete. The reaction temperature was maintained at 4-5°C and the reaction time was 5h. The crude product was poured into 25 mL of saturated saline solution, washed 3 times, dried over anhydrous magnesium sulfate for 30 min, filtered and evaporated under reduced pressure. The target compound was obtained by silica gel column chromatography (ethyl acetate:petroleum ether=1:50).
[0026] The physical properties, yield, nuclear magnetic resonance data and ...
Embodiment 2
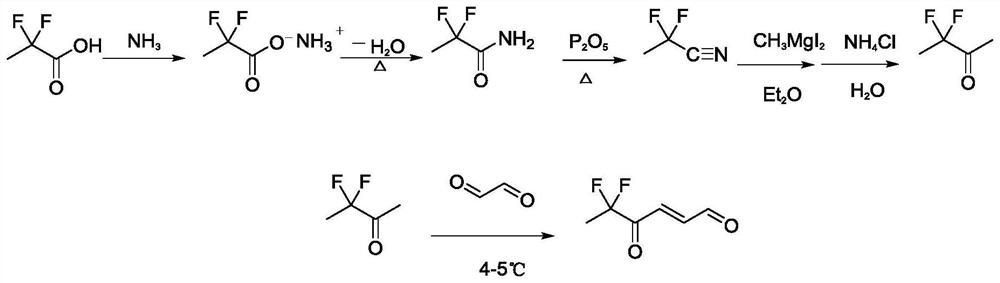
[0028] Example 2 Preparation of 5,5-difluoro-4-oxo-trans-2-hexenal
[0029] The synthetic route of the compound 5,5-difluoro-4-oxo-trans-2-hexenal in the present invention is as follows image 3 shown.
[0030] In a 100 mL three-necked flask, add 2 g (18.18 mmol) of 2,2-difluoropropionic acid and 30 mL of dry methanol solution, replace the air in the three-necked flask with nitrogen, introduce ammonia gas, and conduct the ammoniation reaction for 2 to 4 h under the control of The reaction temperature was room temperature. 1.6 g of 2,2-difluoropropionamide was produced in a yield of 80.8%. 1.6g (14.68mmol) 2,2-difluoropropionamide was dissolved in 25mL toluene solution, in P 2 O 5 Dehydration in the presence of 2,2-difluoropropionitrile. The resulting 2,2-difluoropropionitrile was reacted with methylmagnesium iodide and hydrolyzed to synthesize 3,3-difluoro-2-butanone with a yield of 73.6%.
[0031] Dissolve 1g (9.25mmol) of 3,3-difluoro-2-butanone in 30mL at a concentrat...
Embodiment 3
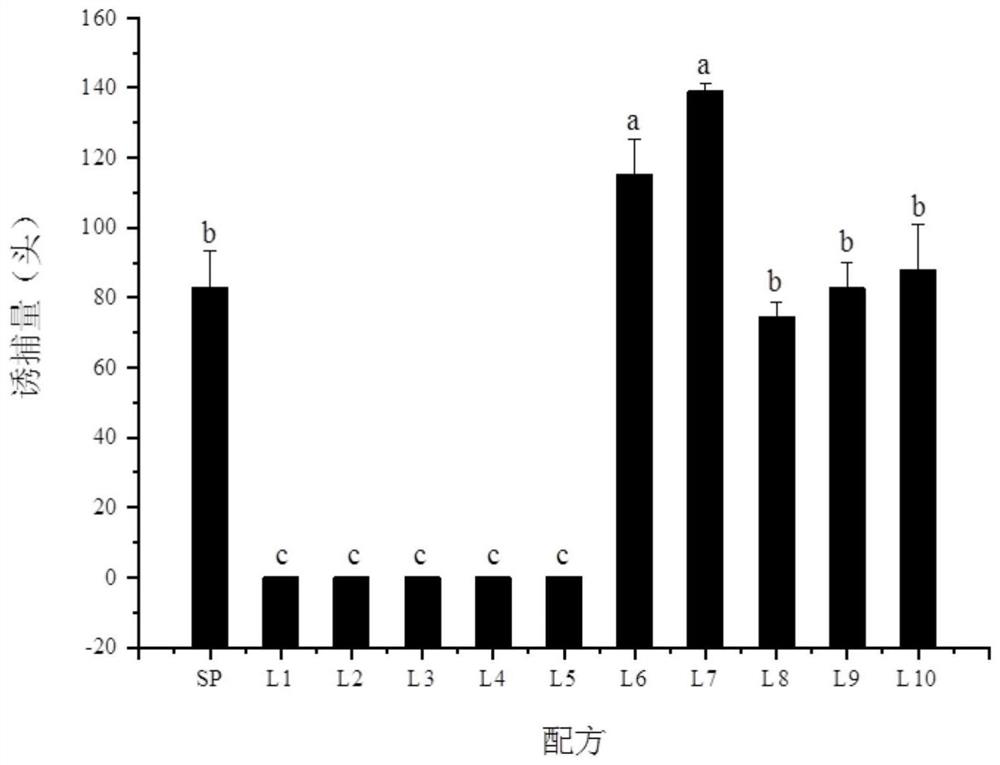
[0035] Embodiment 3 The test of attracting chlorophyll bugs in the field
[0036] The lure cores prepared with the Lygus bug sex attractant composition are placed in boat-type traps, and the traps are placed on the plots where more Lygus bugs are likely to be harmed. In order to ensure the release effect, 100–2000 μg of the chlorophyll sex attractant composition was placed in each 1 mL of the rubber lure. In order to further illustrate the feasibility of this embodiment, 10 specific formulations are given in Table 1.
[0037] Table 1
[0038]
[0039] Select the plots where there may be more chlorotic bug hazards, set traps at a height of about 120cm from the ground for each formula, and the distance between the traps is 10m. Each treatment set up 5 replicates. After placement, the number of trapped green bugs was investigated every other day, and the trapping locations were randomly changed for 20 days. The survey results are shown in Table 2:
[0040] Table 2
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com