Synthesis method and application of 4-oxo-trans-2-hexenal analogue
A synthesis method and hexenal technology are applied in the field of synthesizing sex pheromone analogs, which can solve the problems of insect resistance, short duration, fast release in the field, etc., and achieve long duration and strong stress resistance. , the specificity of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
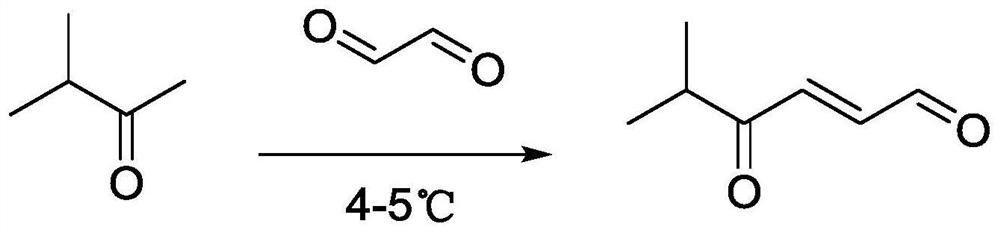
[0023] Example 1 The preparation method of 5-methyl-4-oxo trans-2-hexenal
[0024] The synthetic route of compound 5-methyl-4-oxo trans-2-hexenal in the present invention is as follows figure 2 shown.
[0025] In a 100mL flask, dissolve 1g (11.61mmol) methyl isopropyl ketone in 30mL with a concentration of 0.08mol L -1 Add 0.85 g (14.5 mmol) of glyoxal solution gradually to the sodium hydroxide solution. The reaction process is monitored by TLC. After the raw materials are no longer reduced, the reaction is complete. The reaction temperature is maintained at 4-5° C. and the reaction time is 5 hours. The crude product was poured into 25 mL of saturated saline solution, washed three times, dried over anhydrous magnesium sulfate for 30 min, filtered and rotary evaporated under reduced pressure. Separation on a silica gel column (ethyl acetate:petroleum ether=1:50) gave the target compound.
[0026] The physical properties, yield, NMR data and mass spectrometry data of 5-methy...
Embodiment 2
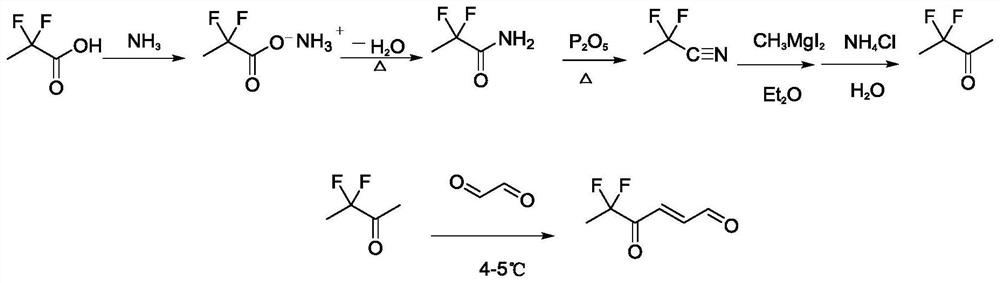
[0028] Example 2 Preparation of 5,5-difluoro-4-oxo-trans-2-hexenal
[0029] The synthetic route of compound 5,5-difluoro-4-oxo-trans-2-hexenal in the present invention is as follows image 3 shown.
[0030] In a 100mL three-necked flask, add 2g (18.18mmol) of 2,2-difluoropropionic acid and 30mL of dry methanol solution, replace the air in the three-necked flask with nitrogen, pass in ammonia gas, and carry out the ammoniation reaction for 2 to 4 hours. The reaction temperature is room temperature. 1.6 g of 2,2-difluoropropanamide was produced, with a yield of 80.8%. 1.6g (14.68mmol) 2,2-difluoropropionamide was dissolved in 25mL toluene solution, in P 2 o 5 In the presence of dehydration into 2,2-difluoropropionitrile. 3,3-difluoro-2-butanone can be synthesized by reacting the generated 2,2-difluoropropionitrile with methylmagnesium iodide followed by hydrolysis, with a yield of 73.6%.
[0031] The concentration of 1g (9.25mmol) 3,3-difluoro-2-butanone dissolved in 30mL ...
Embodiment 3
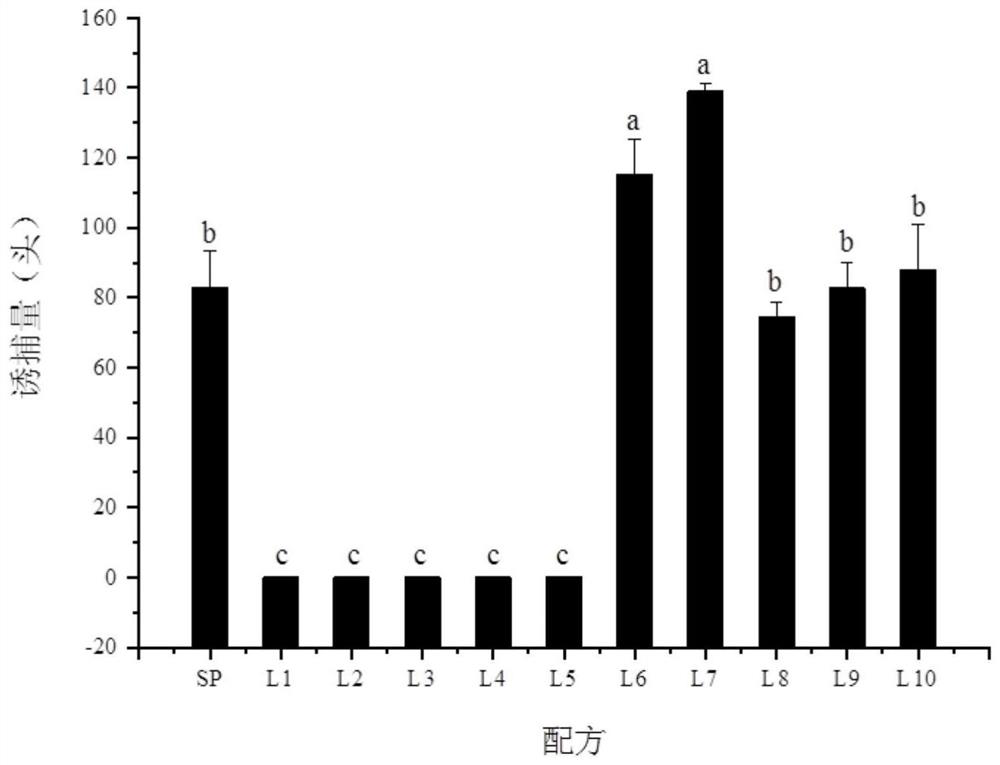
[0035] Embodiment 3 field lures the green Lygus test
[0036] The lure cores prepared with the lucid lygus sex attractant composition are placed in boat-type traps, and the traps are placed in plots that may cause more harm to livid bugs. In order to ensure the release effect, 100-2000 μg of the lucid bug sex attractant composition is placed in every 1 mL of the rubber lure core. In order to further illustrate the feasibility of the present embodiment, 10 specific formulas are provided in Table 1.
[0037] Table 1
[0038]
[0039] Select the plots that may cause more harm of Lygus lygus, each formula sets traps about 120cm above the ground, and the distance between traps is 10m, randomly place the traps containing the above-mentioned Lygus sex pheromone composition, every Five replicates were set up for each treatment. The number of trapped Lygus spp. was surveyed every other day after placement, and the trapping location was changed randomly, and the survey continued f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com