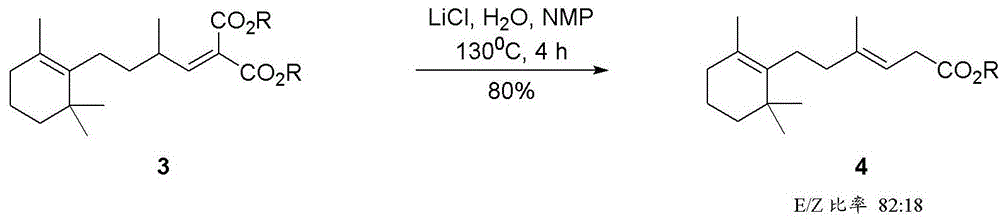
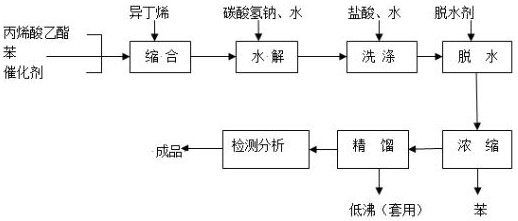
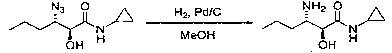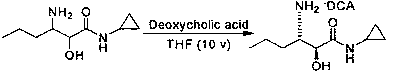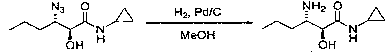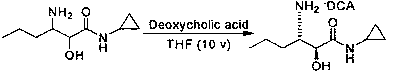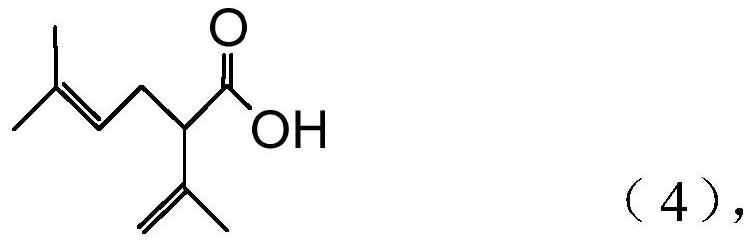Patents
Literature
44 results about "Hexacosenoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
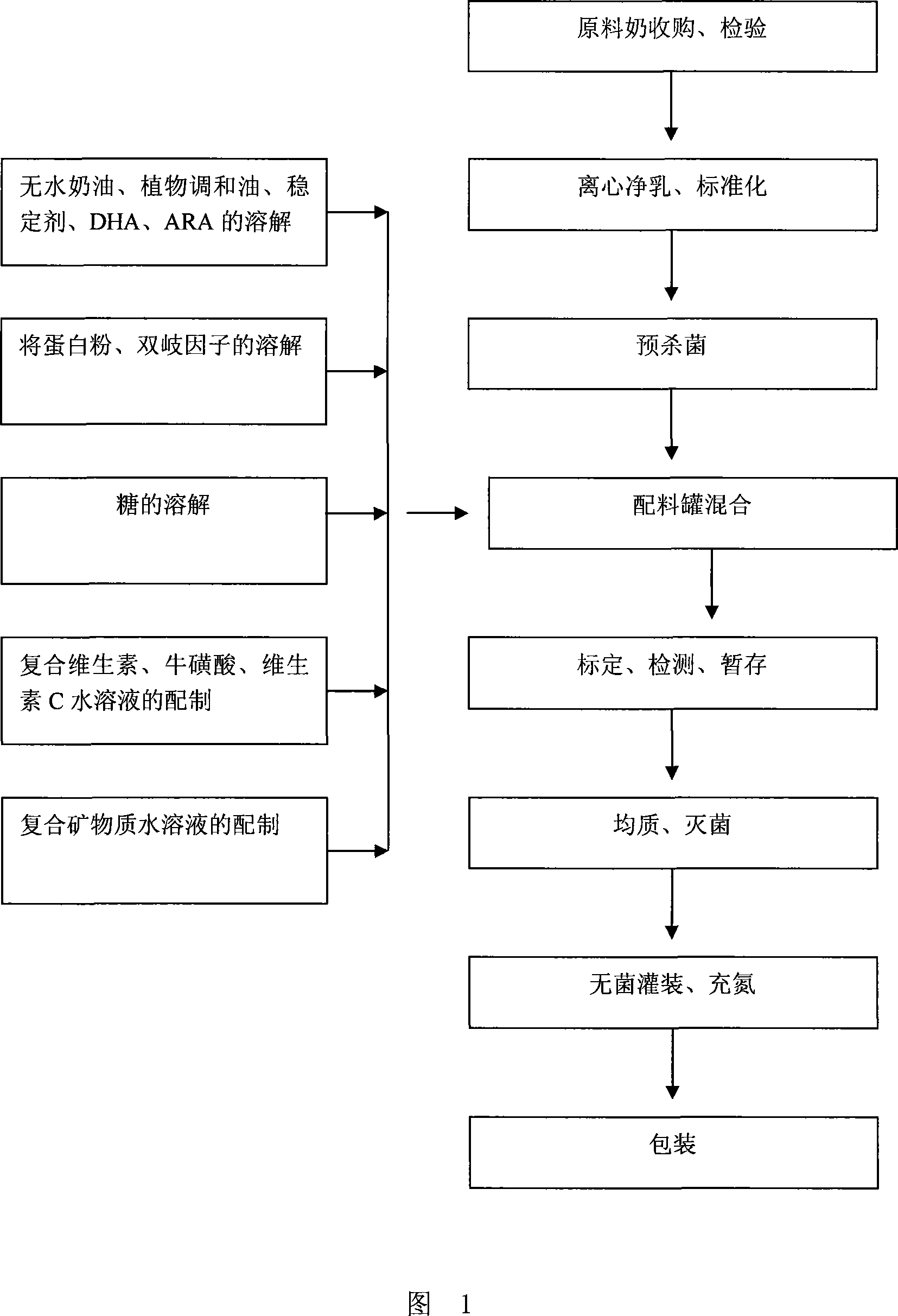
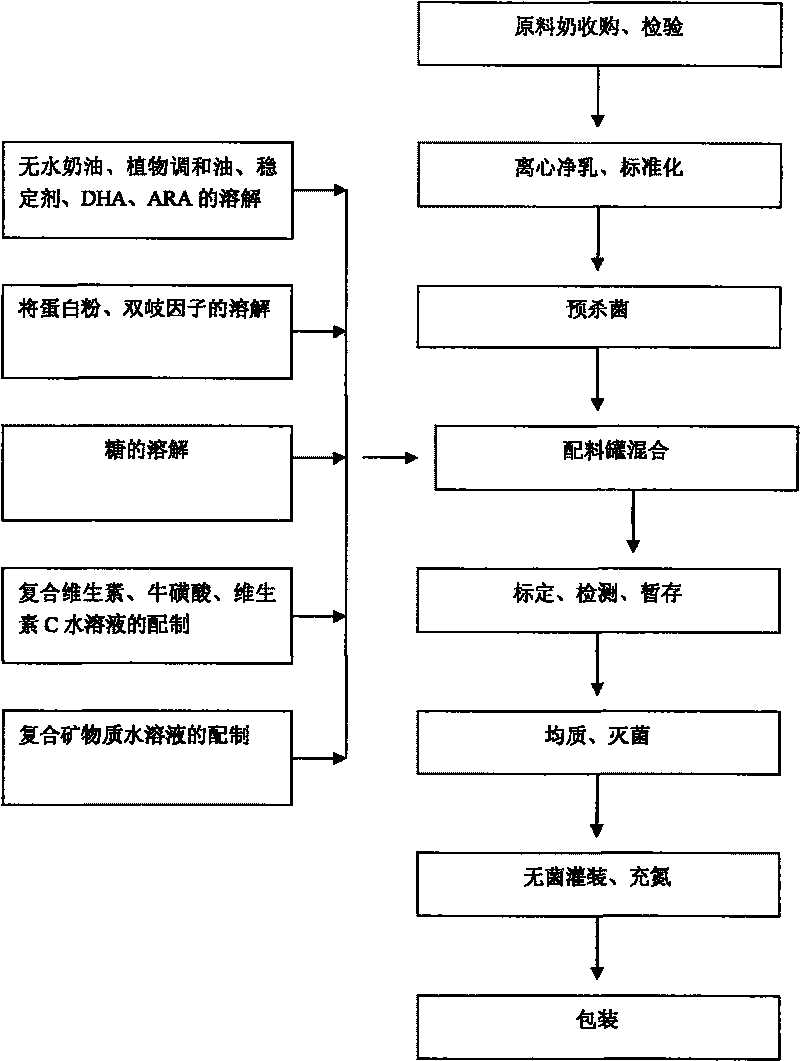
Liquid milk suitable for 1 to 3 years old children and the method for producing the same
The invention discloses a kind of liquid milk and the method for preparing the same. Said liquid milk comprises following raw materials and the weight of them are as follows: milk 30-70g, sugar 5-9g, waterless cream 0.59-2.27g, protein powder 0.05-1.375g, plant mixed oil 0.11-1.35g, stabilizer 0.1-0.4g, isomaltose hypgather 0.1-0.8g, complex vitamin 0.018-0.05g, mineral matter 0.025-0.065g, vitamin C 5-50g, taurine 0.8-6.83g, hexacosenoic acid 1-5g, arachidonic acid 2-8g and pure water 100g. The liquid milk is suitable for baby of 1-3 years old, it contains protein, fat, carbohydrate, vitamin and mineral matter for growth, and the content and proportion are scientific, which in coincidence with baby growth features.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Application of mo-based catalyst loaded by unreduced or partially reduced polymetallic oxide in preparation of organic chemical product with lignin
InactiveCN107759444AHigh catalytic activityWide variety of sourcesOxygen-containing compound preparationOrganic compound preparationOrganic chemicalsEthyl caproate
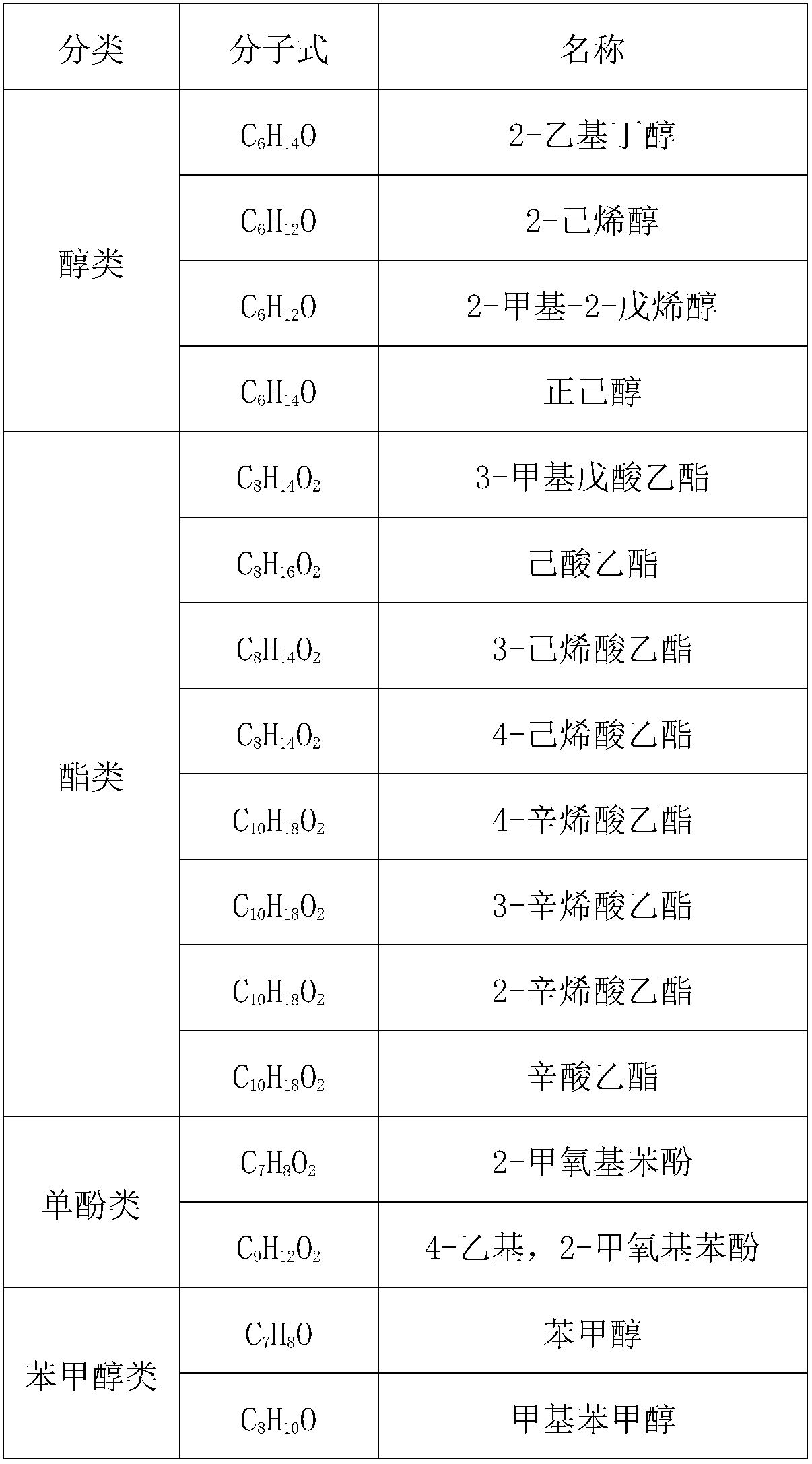
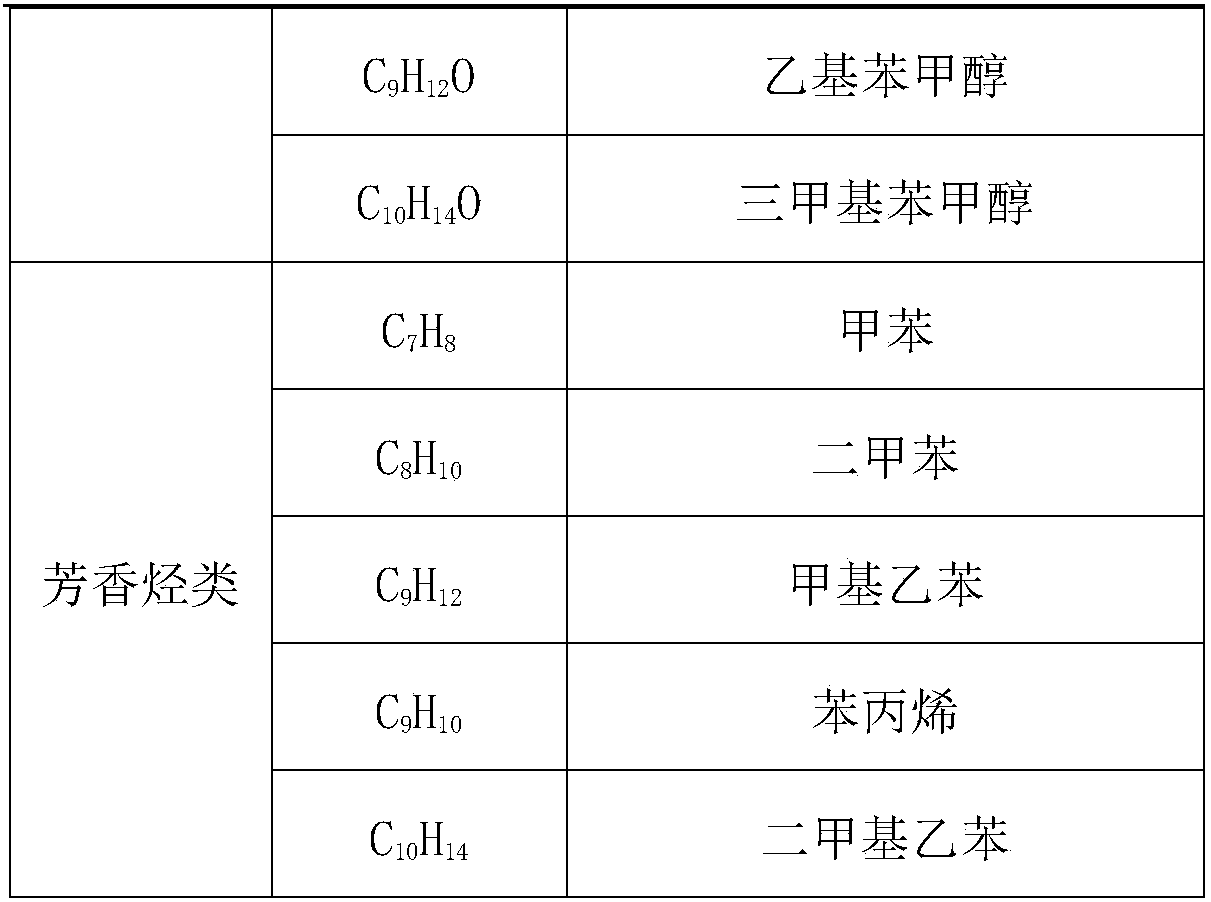
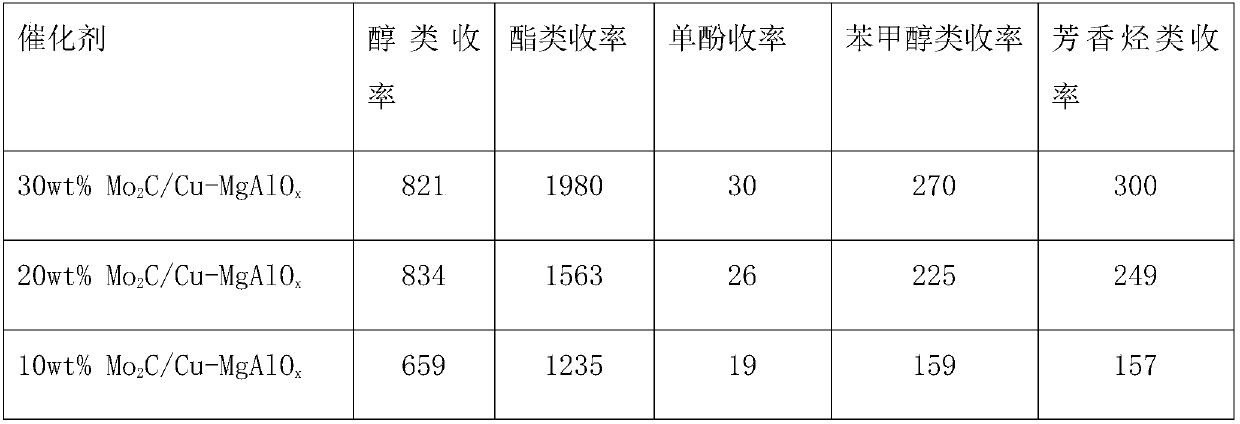
The invention provides application of a mo-based catalyst loaded by an unreduced or partially reduced polymetallic oxide in preparation of an organic chemical product with lignin. The application comprises the steps of after the lignin, the catalyst and a reaction solvent are mixed, adding a mixture into a sealed reaction container, introducing gas into the container, increasing the temperature at230-350 DEG C, stirring the mixture and making the mixture react for 0.5-12 h, after reaction, filtering out the catalyst, conducting rotary evaporation, and obtaining the liquid product. The application of the mo-based catalyst loaded by the unreduced or partially reduced polymetallic oxide in preparation of the organic chemical product with the lignin has the advantages that the catalyzing process has a high yield of product, on the optimized reaction condition, the total mass yield of a micromolecule organic product reaches 349%, which is because solvent molecules and lignin depolymerizedsmall molecules are combined further to generate useful molecules such as ethyl caproate, ethyl cis-3-hexenoate, 4-ethyl cis-3-hexenoate, ethyl 3-methylvalerate, 3-ethyl trans-2-octenoate and ethyl caprylate, the product contains aromatic compounds such as monophenol applied to industry in large amounts, the additional value of the catalyst is high, and the catalyst has a good industrial application prospect.
Owner:TIANJIN UNIV +1
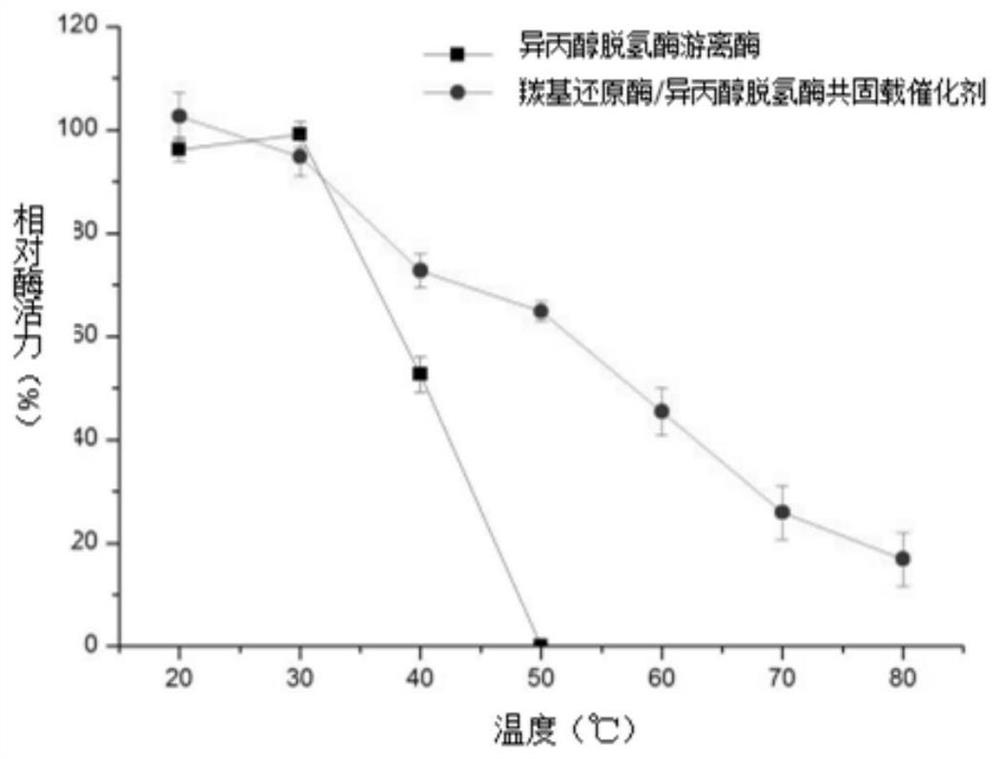
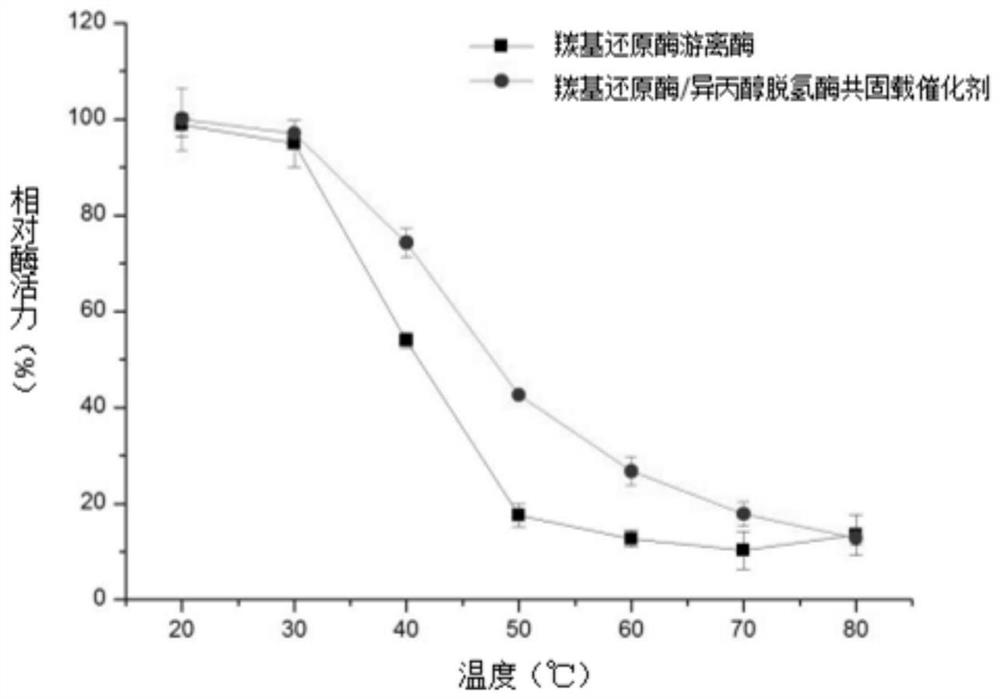
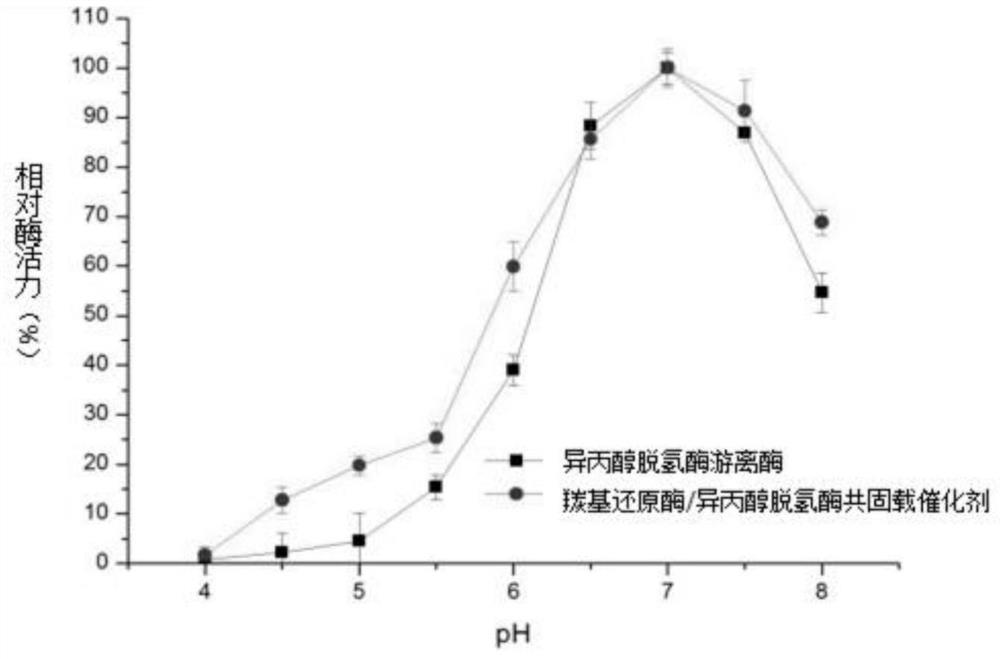
Carbonyl reductase/isopropanol dehydrogenase co-immobilized catalyst as well as preparation method and application thereof
PendingCN111686809AHigh catalytic efficiencyImprove stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHexacosenoic acidPtru catalyst
The invention belongs to the technical field of biological pharmacy, and particularly discloses a carbonyl reductase / isopropanol dehydrogenase co-immobilized catalyst as well as a preparation method and application thereof. According to the invention, carbonyl reductase and isopropanol dehydrogenase are co-embedded in a solid phase carrier obtained from polyvinyl alcohol and polyethylene glycol; the obtained co-immobilized enzyme catalyst can realize efficient and high stereoselectivity reduction of 3-carbonyl-5-hexenoic acid ester to generate (R)-3-hydroxy-5-hexenoic acid ester through catalysis of carbonyl reductase and circulation of isopropanol dehydrogenase on coenzyme NADPH. The catalyst can be used for preparing (R)-3-hydroxy-5-hexenoic acid ester by taking 3-carbonyl-5-hexenoic acid ester as a substrate, and the co-immobilized catalyst prepared by the method disclosed by the invention is high in catalytic efficiency, good in stability, reusable, concise in process and low in cost, and has excellent actual industrial application value.
Owner:FUDAN UNIV
Method for synthesizing racemic sclareolide
InactiveCN102351822AEnvironmentally friendlyEasy to operateOrganic chemistryHexacosenoic acidPtru catalyst
The invention belongs to the technical field of daily use chemicals, and discloses a method for synthesizing racemic sclareolide. The synthetic method comprises the step of: making 4-methly-6-(2,6,6-trimethyl-1-cyclohexenyl)-3-hexene-1-acid serving as a raw material react with a solid super acid serving as a catalyst in an organic solvent to obtain racemic sclareolide. The method for synthesizing the racemic sclareolide is environmentally-friendly, is easy to operate, and has high yield; and the catalyst used in the synthetic method can be used repeatedly, so that the production cost is lowered, and the method is more suitable for industrial production.
Owner:北京安胜瑞力科技有限公司
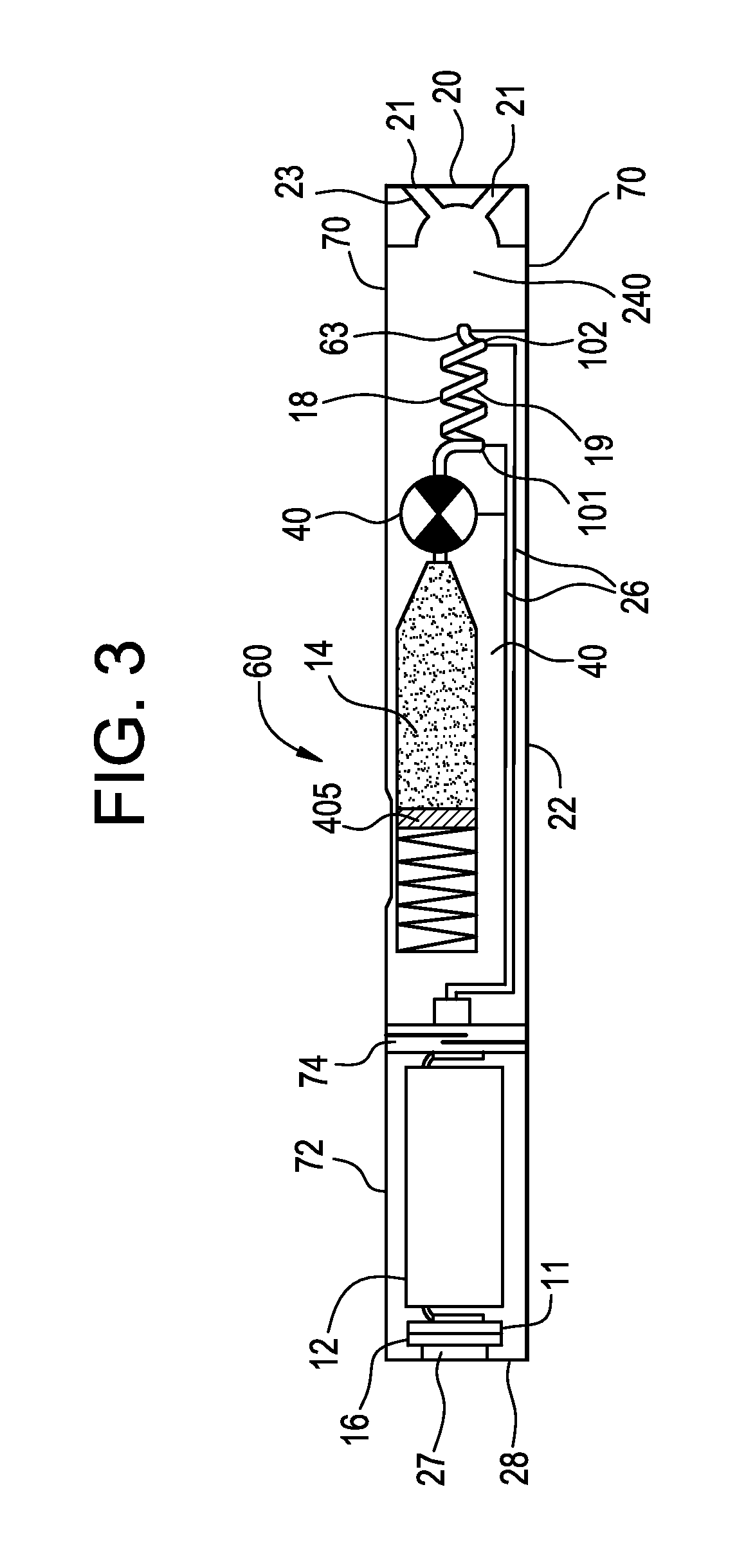
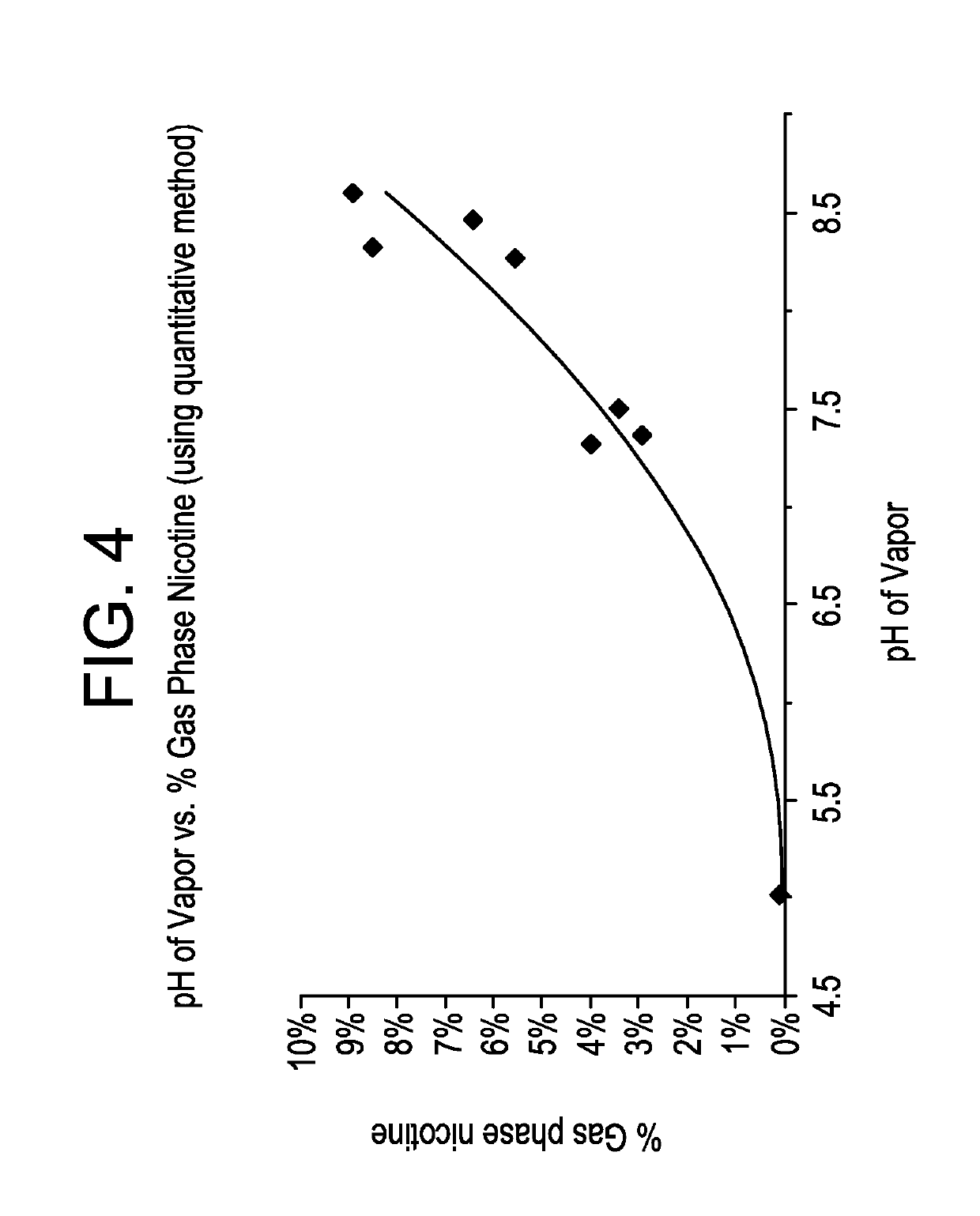
Pre-vaporization formulation for controlling acidity in an e-vaping device
ActiveUS10327472B2Increase acidityGreat proportionTobacco treatmentPharmaceutical delivery mechanismBenzoic acid4-pentenoic acid
Owner:AKRIA CLIENT SERVICES LLC
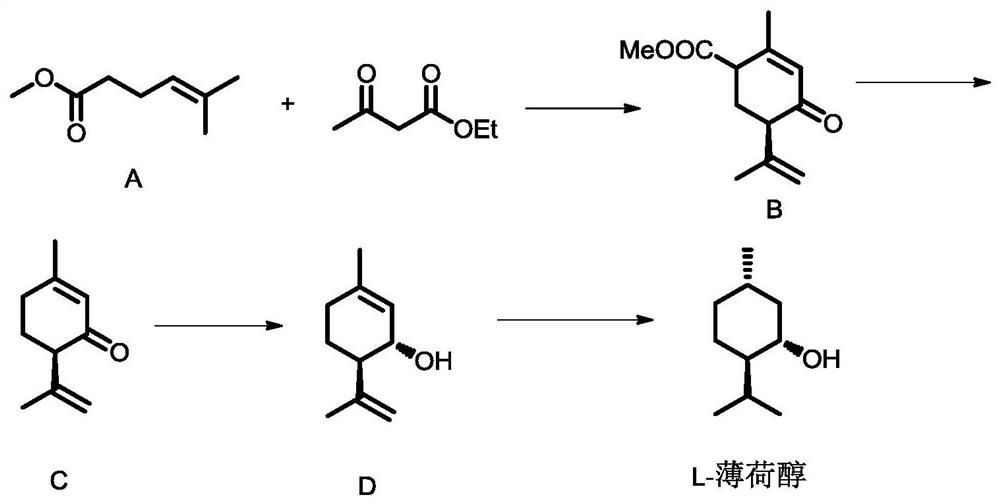
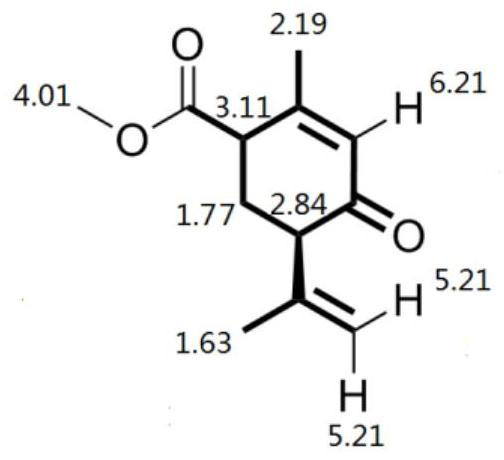
Preparation method of optically active menthol
ActiveCN112573996AHigh yieldLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHexacosenoic acidPtru catalyst
The invention provides a preparation method of optically active menthol, wherein the preparation method comprises the following steps: 1) carrying out alkali pretreatment on a compound A (methyl 5-methyl-4-hexenoate), and cyclizing with ethyl acetoacetate under the action of a copper salt chiral phosphine catalyst to generate a compound B (methyl isomenthodienone-4-formate); 2) carrying out a decarboxylation reaction on the compound B under the action of alkali to generate a compound C (isomenthodienone); 3) reducing the compound C under the action of a catalyst to generate a compound D (isomenthyl dienol); and 4) hydrogenating the compound D under the action of chiral induction and a catalyst to generate the optically active menthol. The preparation method disclosed by the invention has the advantages of novel reaction route, readily available raw materials, low price and mild reaction conditions, and is suitable for industrial production.
Owner:WANHUA CHEM GRP CO LTD
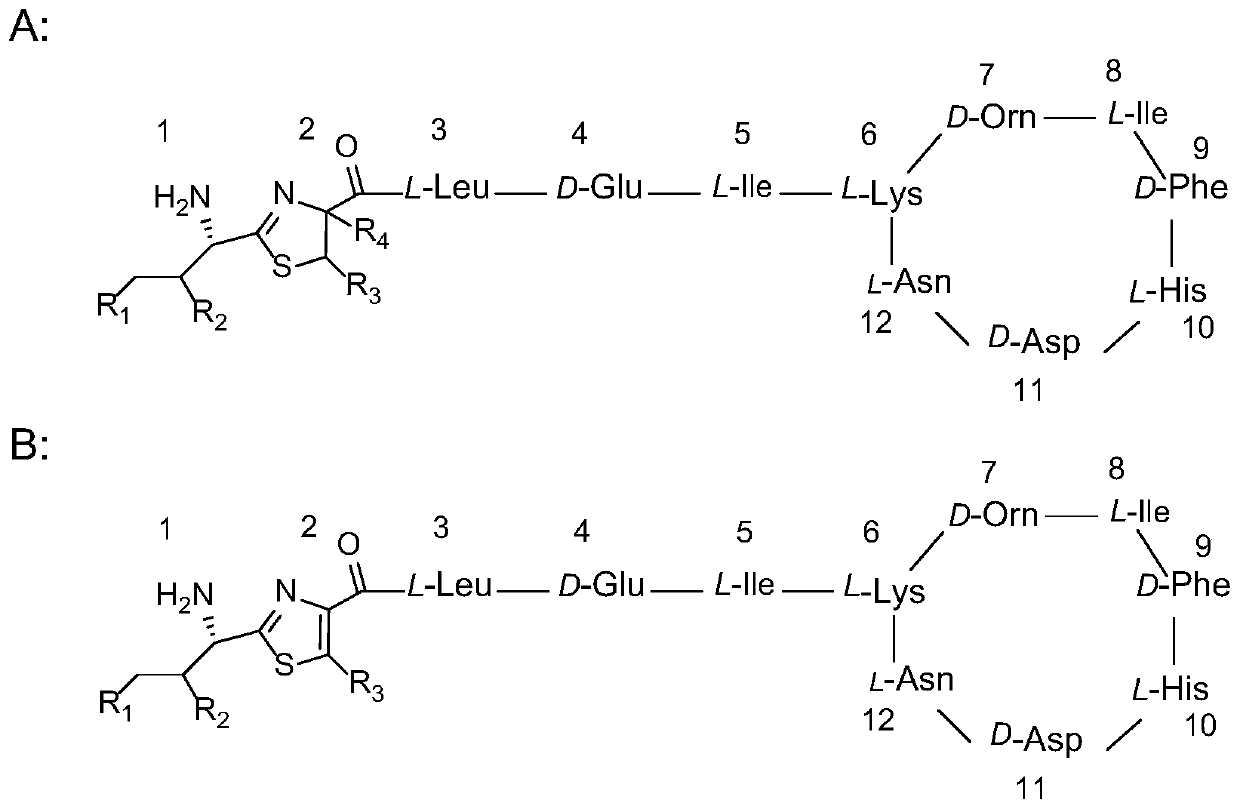
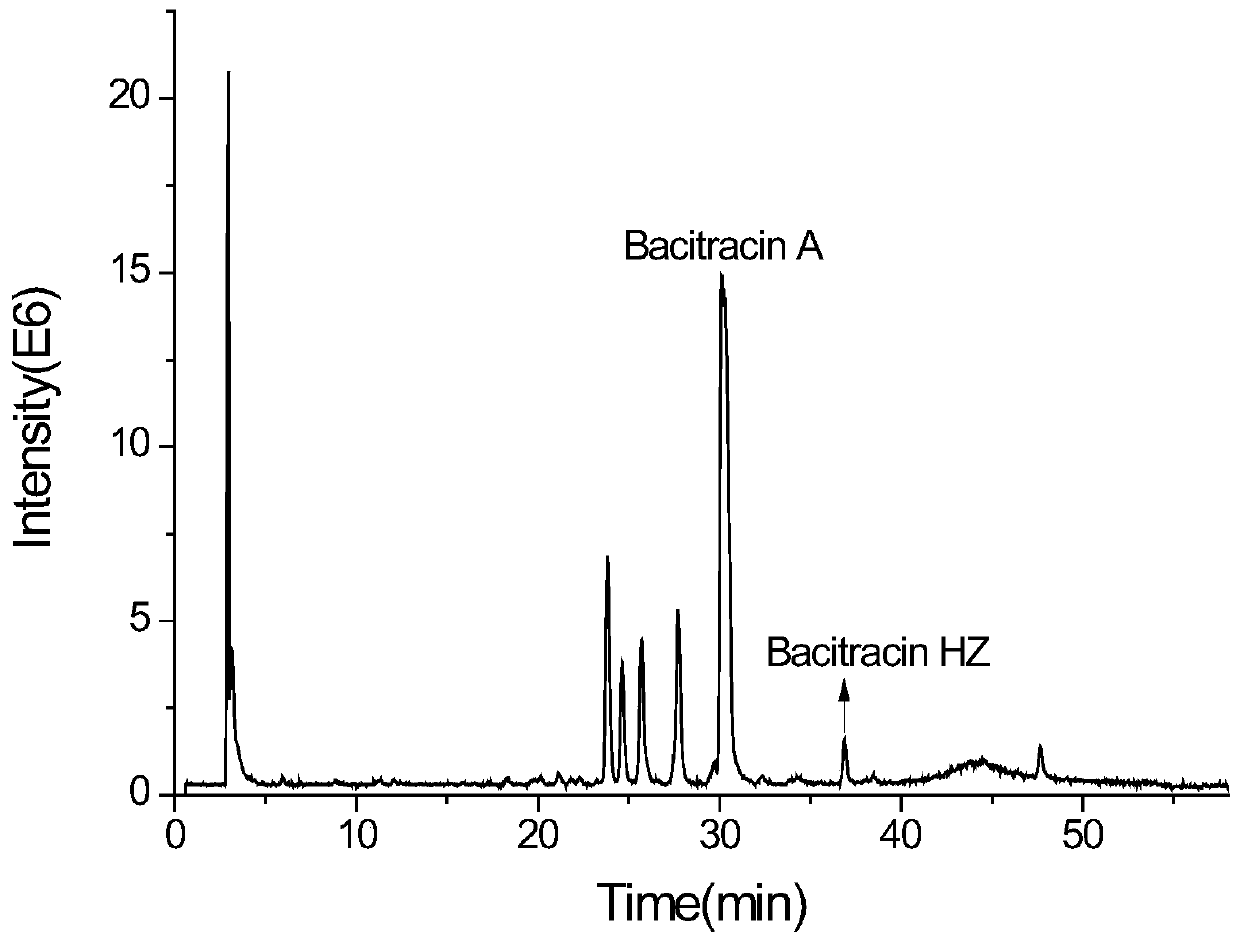
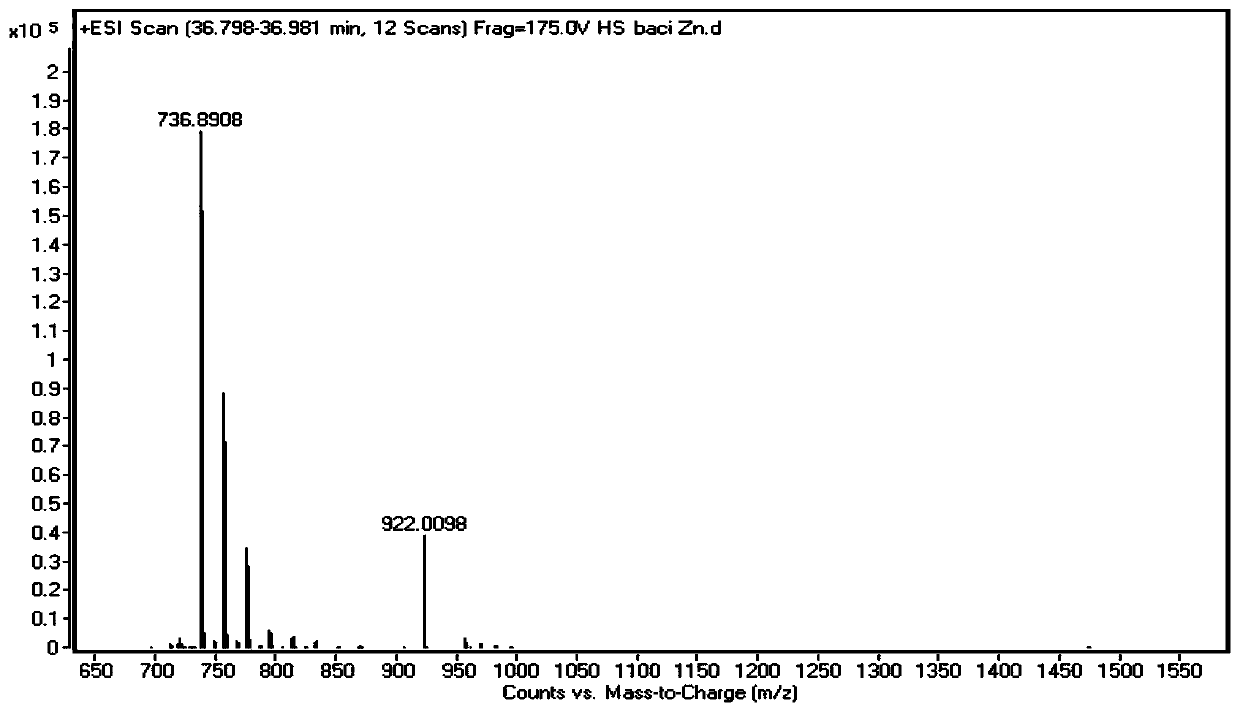
Analysis method of bacitracin and components thereof
The invention discloses a novel bacitracin (Bacitracin) component and a high performance liquid chromatography-mass spectrometry (HPLC-MS) analysis method thereof. The component is verified to be bacitracin A subjected to double methylenation and vinylation or bacitracin Y subjected to double methylenation and ethylation through HPLC-MS (High Performance Liquid Chromatography-Mass Spectrometry) and secondary mass spectrometry, and the novel component is named Bacitracin HZ. The invention discloses the presence of novel non-protein amino acids, such as 2-amino-3-vinyl-5-hexenoic acid, 2-amino-4-vinyl-5-hexenoic acid, 3-vinylcysteine, 2-vinylcysteine and the like. The bacitracin HZ has antibacterial activity or toxicity different from antibacterial activity or toxicity of common bacitracin components and has potential application value; and the structure confirmation of the compounds also provides a research basis for strictly controlling the quality of bacitracin.
Owner:SHANGHAI INST FOR FOOD & DRUG CONTROL
Amhexenoic acid preparation liquid composition
PendingCN114642633AImprove medication safetyImprove complianceOrganic active ingredientsNervous disorderHexacosenoic acidPatient compliance
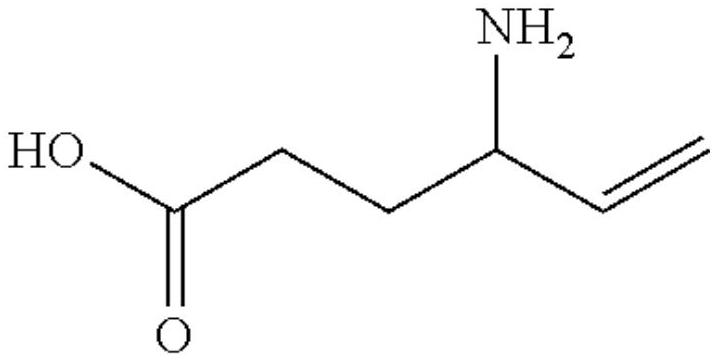
The invention discloses an amhexenoic acid preparation liquid composition and also discloses an oral solution prepared from the composition and a diluent, and the solution is suitable for treating CNS diseases, such as patients with resistant epilepsy, complex partial epileptic seizure, secondary systemic epileptic seizure, refractory complex partial seizure and infant spasm. The dosage form capable of being used for multiple times is good in stability, convenience is provided for medical staff, patient compliance is improved, human errors during dosage are reduced, and the medication safety of children and babies is also improved.
Owner:SHANGHAI AUCTA PHARMA CO LTD
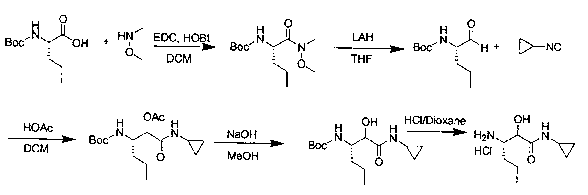
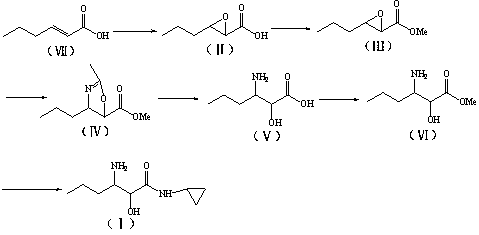
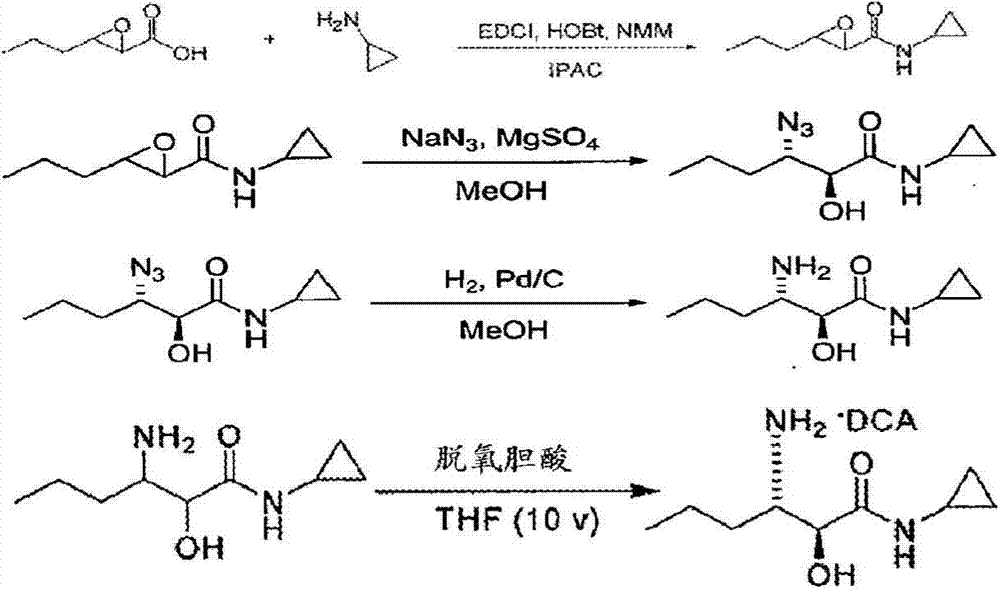
Preparation method of (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride
InactiveCN102702015AEasy to operateMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationHexacosenoic acidPharmaceutical Substances
The invention discloses a preparation method of (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride, and belongs to the technical field of preparation of a medical intermediate. The chiral product of the (2S, 3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride is obtained by performing the five reaction steps, including epoxidation, resolution, ring opening, esterification and amination, on trans-2-hexenoic acid serving as an initial raw material, wherein water is used as a reaction solvent in the first step of epoxidation. By adoption of the technology, the preparation method has the advantages of convenience in operation, mild reaction conditions, cheap and readily available used raw materials, low toxicity, high product purity reaching over 99.4 percent, high e.e. (Enantionmeric Excess) value reaching over 99.5 percent, low equipment requirement and suitability for large-scale industrial production.
Owner:ZHEJIANG LANGHUA PHARMA
Liquid milk suitable for 1 to 3 years old children and the method for producing the same
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Method for preparing 3-amino-N-cyclopropyl-2-hydroxyhexanamide
InactiveCN102702016AEasy to operateMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationHexacosenoic acidBiochemical engineering
The invention discloses a method for preparing 3-amino-N-cyclopropyl-2-hydroxyhexanamide, and belongs to the technical field of the preparation of medicinal intermediates. The method comprises the step that 3-amino-N-cyclopropyl-2-hydroxyhexanamide is prepared from trans-2-hexenoic acid by epoxidation, esterification, reduction and other steps. The method has readily-available raw materials and stable process condition, and is suitable for scale industrial production. The 3-amino-N-cyclopropyl-2-hydroxyhexanamide is conveniently prepared from readily-available and low-toxicity raw materials by the step above under a mild reaction condition; the total yield is as high as above 61%, the product has high purity which is as high as above 99.4%; and furthermore, the method achieves high yield and a high-purity product, has a low requirement on the equipment and is suitable for industrial production.
Owner:ZHEJIANG LANGHUA PHARMA
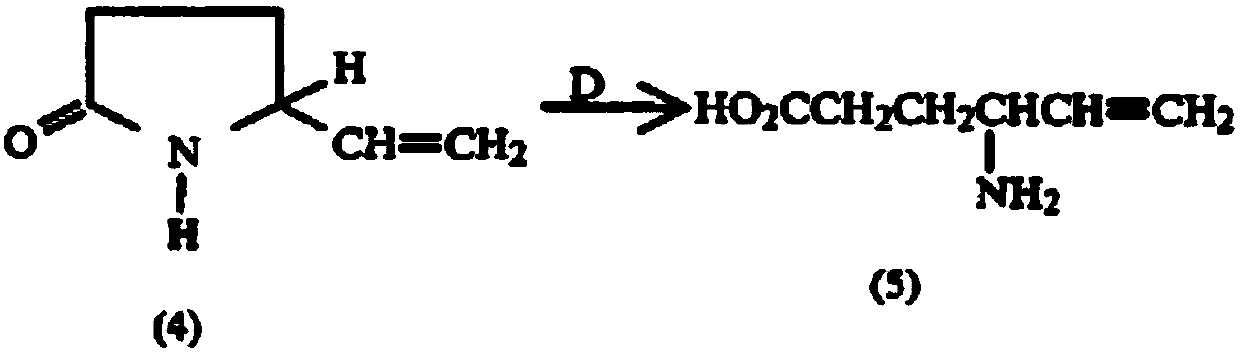
Preparation method of aminohexenoic acid
ActiveCN110713440AHigh purityEasy to operateGroup 4/14 element organic compoundsOrganic compound preparationHexacosenoic acidHydrolysis
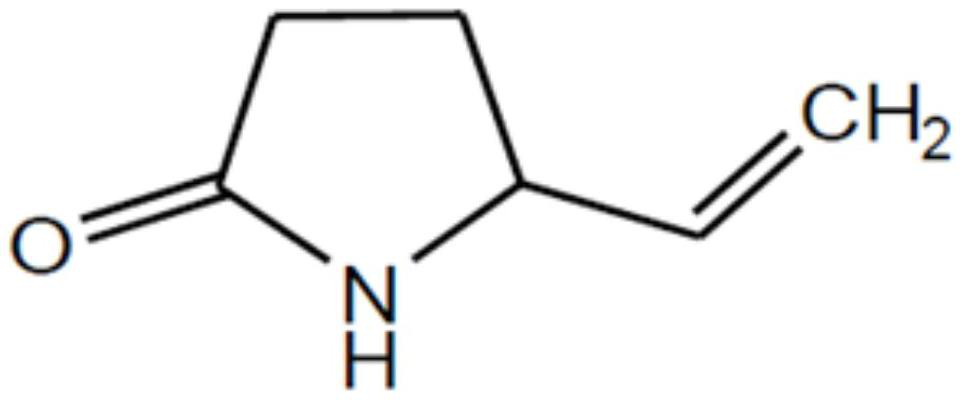
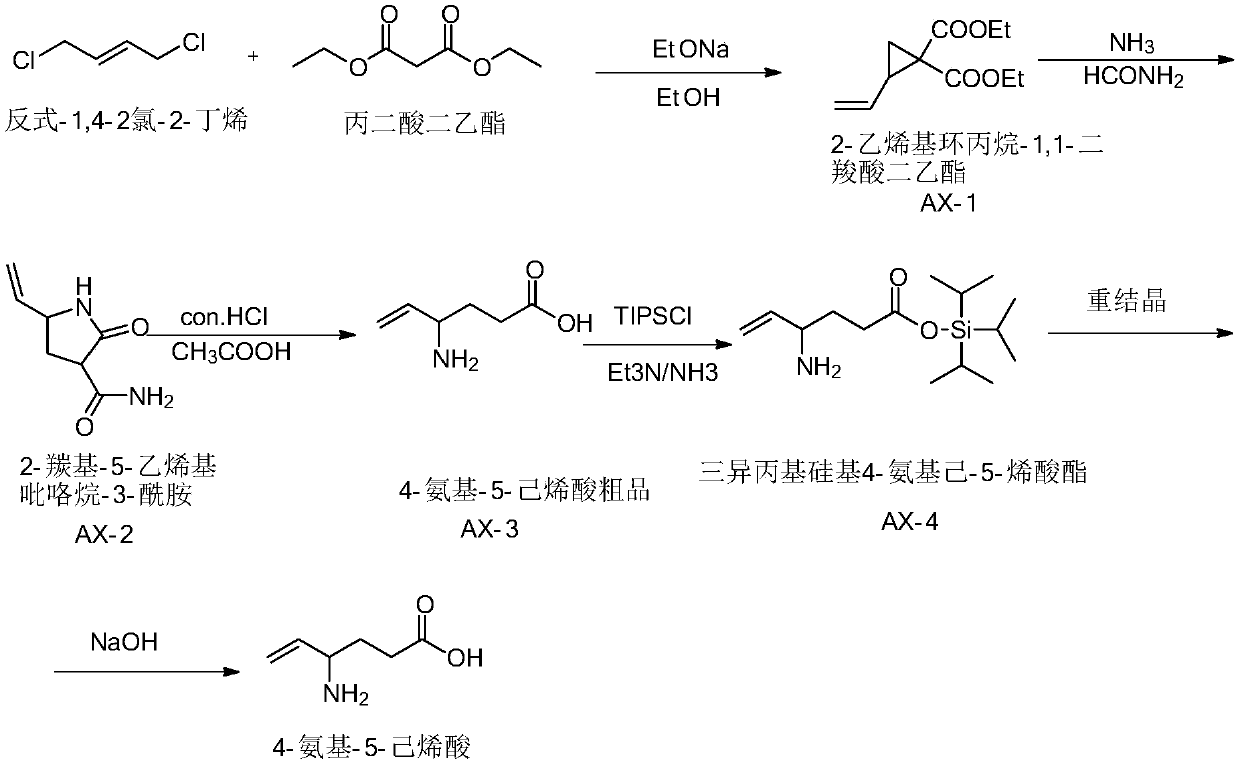
The invention discloses a preparation method of aminohexenoic acid, which is characterized by comprising the following steps: with diethyl malonate and 1,4-dichloro-2-butene as raw materials, preparing 2-vinylcyclopropane-1,1-dicarboxylic acid diethyl ester; then carrying out a reaction on 2-vinylcyclopropane-1,1-dicarboxylic acid diethyl ester in the presence of ammonia and formamide to prepare 2-carbonyl-5-vinyl-pyrrolidine-3-amide; preparing a 4-amino-5-hexenoic acid crude product from the 2-carbonyl-5-vinyl-pyrrolidine-3-amide under an acidic condition; then preparing triisopropylsilyl-4-aminohexyl-5-alkenyl ester from 4-amino-5-hexenoic acid and triisopropylchlorosilane in the presence of triethylamine and ammonia; recrystallizing the triisopropylsilyl-4-aminohexyl-5-alkenyl ester inchloroform / methanol to obtain a purified product of the triisopropylsilyl-4-aminohexyl-5-alkenyl ester; wherein the volume ratio of chloroform to methanol is 1:7; and hydrolyzing the purified productof the triisopropylsilyl-4-aminohexyl-5-alkenyl ester under an alkaline condition to obtain the aminohexylenic acid.
Owner:WUHAN WUYAO SCI & TECH
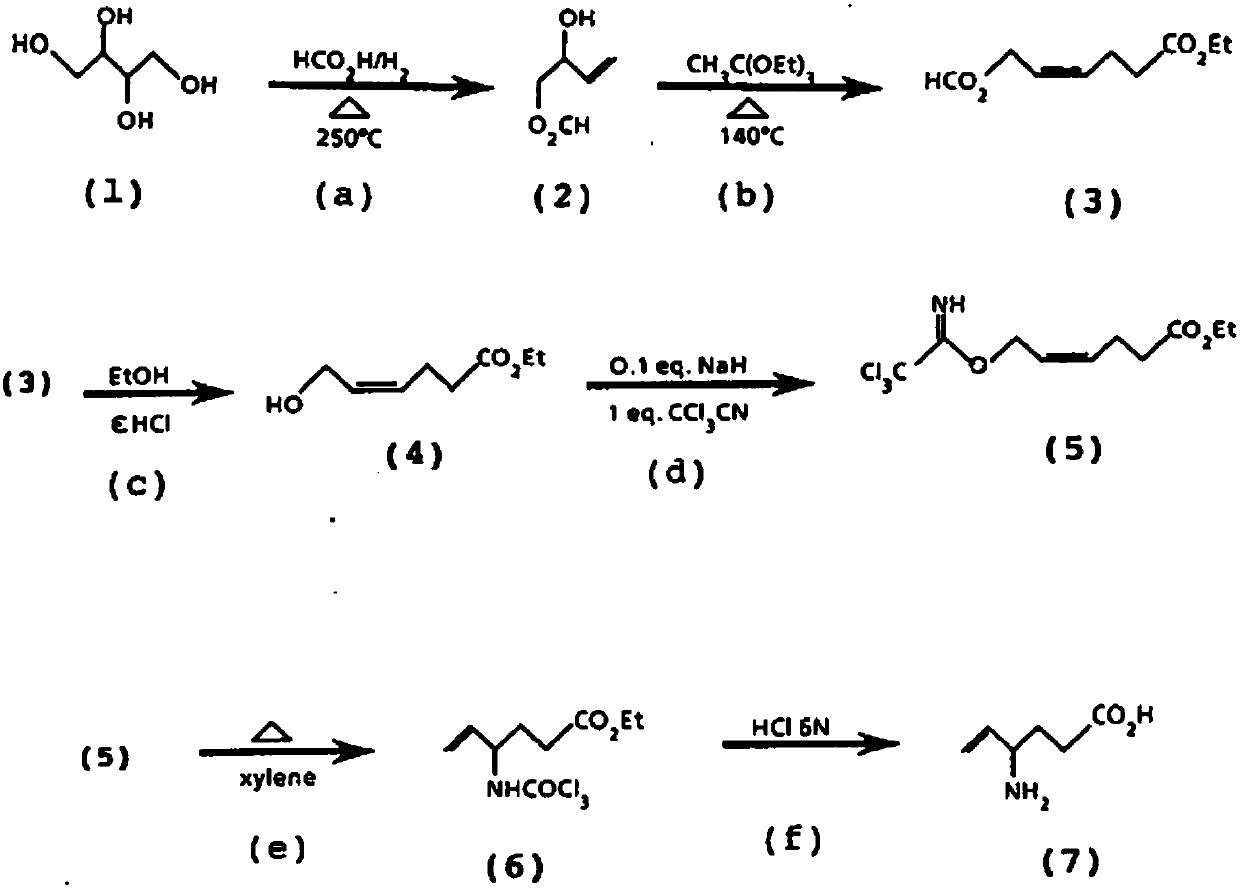
Synthesis method for trans-3-hexenoic acid
InactiveCN102838474ANo residueReduce the introductionOrganic compound preparationCarboxylic preparation by ozone oxidationHexacosenoic acidPtru catalyst
The invention discloses a synthesis method for trans-3-hexenoic acid. According to the synthesis method, butyl aldehyde and malonic acid are used as starting raw materials, and triethanolamine is used as a catalyst. The synthesis method comprises the following steps of: preparing mixed solution, i.e., uniformly mixing and stirring the triethanolamine and the malonic acid according to the proportion of 1:3-3:1; performing dropping reaction, i.e., stirring and dropwise adding the mixed solution into the butyl aldehyde, charging N2 to protect the butyl aldehyde, heating to 60-100 DEG C, and separating by using an oil-water separator to remove water produced by the reaction, wherein the reaction time is 2-4 hours; and fractionating, i.e., removing the butyl aldehyde and front cut fraction through fractionation and then evaporating out a finished product. The trans-3-hexenoic acid synthesized by using the method has the characteristics of less isomer, mild reaction, simple process, high yield and no solvent residue.
Owner:TIANNING FLAVOR JIANGSU
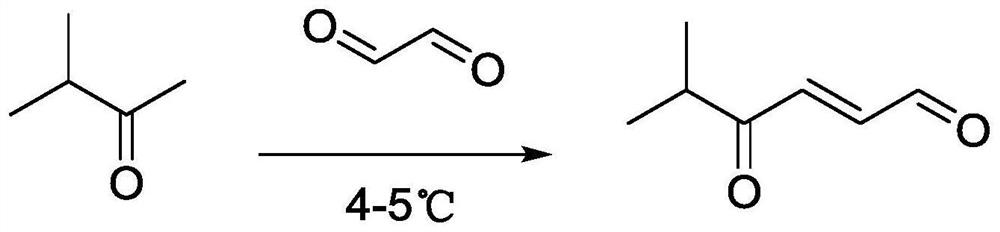
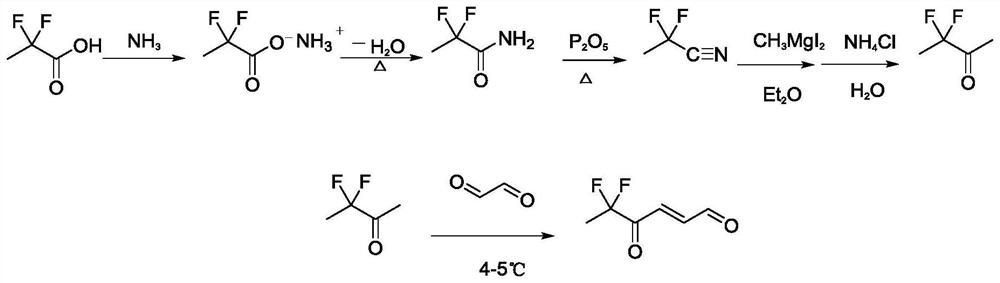
Synthesis method and application of 4-oxo-trans-2-hexenal analogue
ActiveCN112094181AHigh trapping activityImprove stabilityPreparation by organometalhalide reactionBiocideHexacosenoic acidMethyl palmoxirate
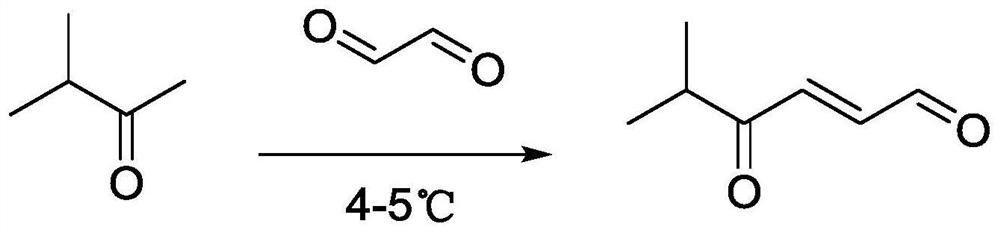
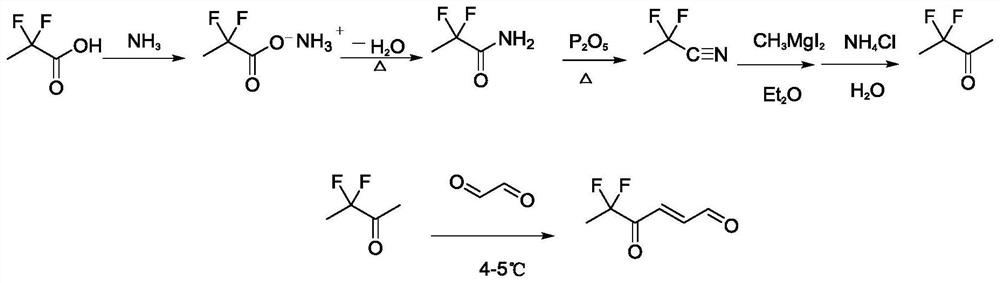
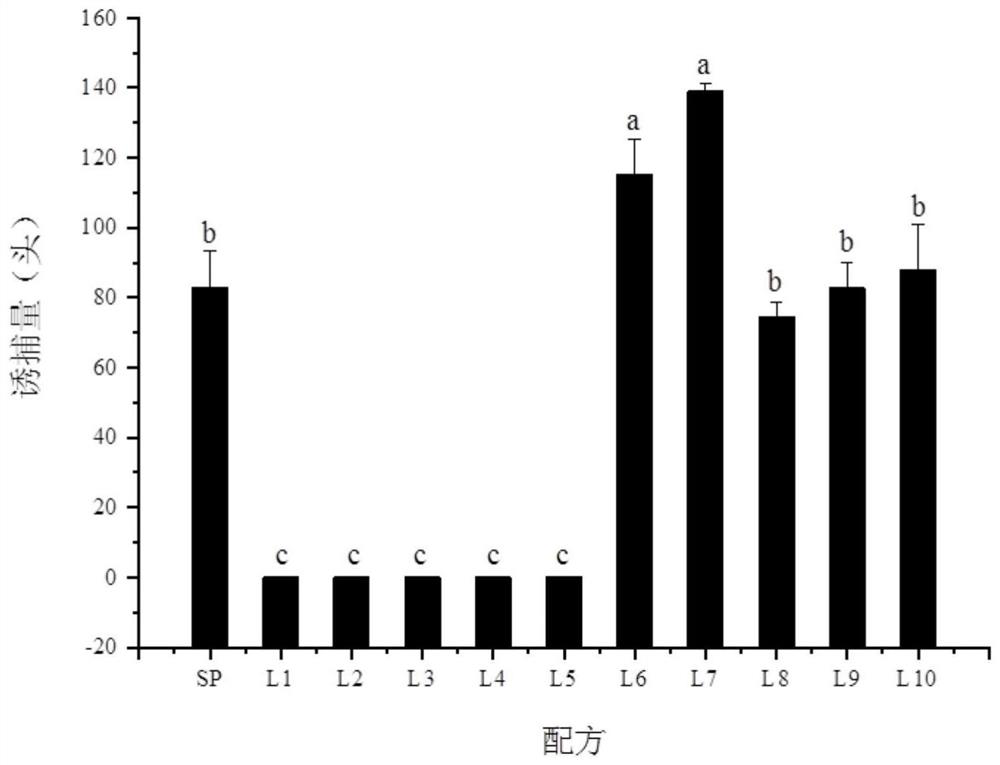
The invention discloses a synthesis method and application of a 4-oxo-trans-2-hexenal analogue, and belongs to the technical field of biological prevention and control. The analogues comprise 5-methyl-4-oxo-trans-2-hexenal, 5, 5-difluoro-4-oxo-trans-2-hexenal, 4-oxo-trans-2-hexenoic acid, 4-oxo-trans-2-hexenol and 4-hydroxy-trans-2-hexenal. One or more of the five compounds completely replace 4-oxo-trans-2-hexenal in sex pheromone components of apolygus lucorum, lygus pratensis and apolygus lucorum. The synthesis methods of the 5-methyl-4-oxo-trans-2-hexenal and the 5, 5-difluoro-4-oxo-trans-2-hexenal are found for the first time, the application composition can significantly reduce the release rate of the lure, prolong the trapping time and save the cost, the problem that the 4-oxo-trans-2-hexenal is unstable in the field can be avoided, and the application range of the 5-methyl-4-oxo-trans-2-hexenal is widened. Male insects such as apolygus lucorum, herbage miridae and apolygus lucorum can be trapped and killed more effectively.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Process for preparing unsaturated esters
ActiveCN104557543BOrganic compound preparationCarboxylic acid esters preparationHexacosenoic acidMalonic acid
The present invention provides a method for preparing 4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hex-3-enoic acid alkyl ester (4), the method comprising making 2 ‑Methyl‑4‑(2,6,6‑trimethylcyclohex‑1‑en‑1‑yl)butyraldehyde (1) in a single step reaction with monoalkyl malonate (5); where R selected from H, C1-C4 alkyl.
Owner:AROMOR FLAVORS & FRAGRANCES LTD
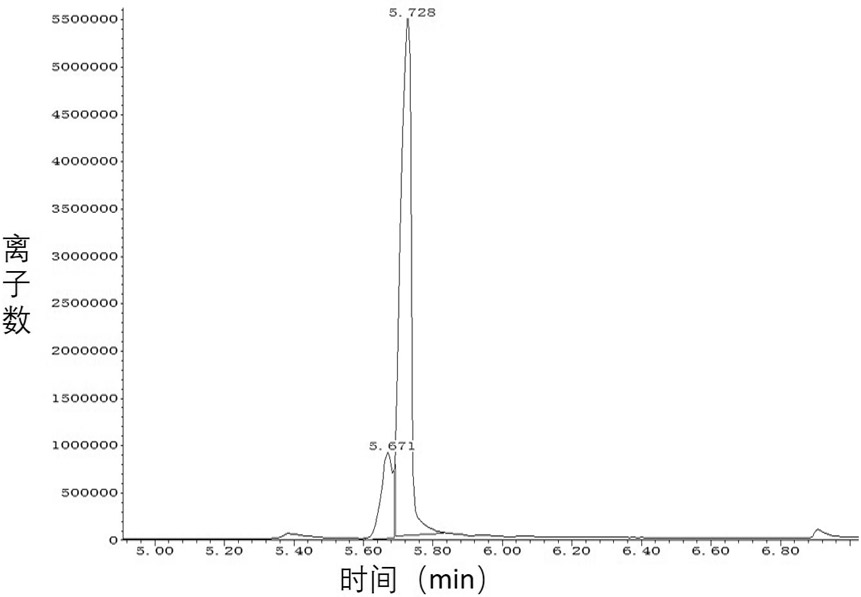
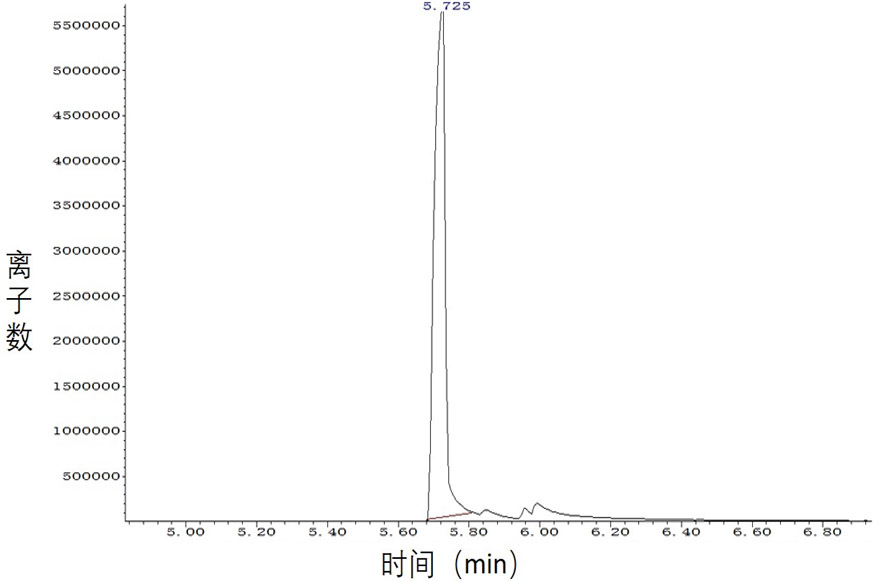
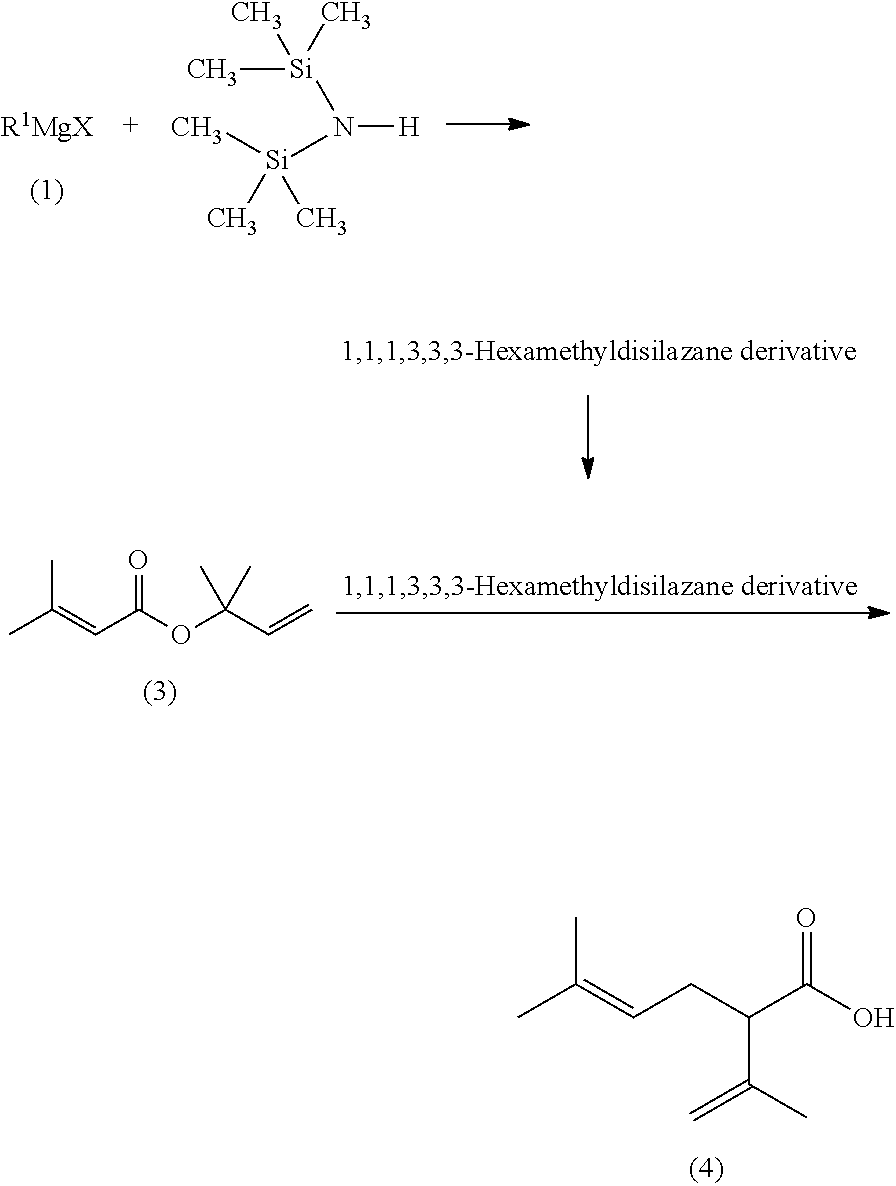
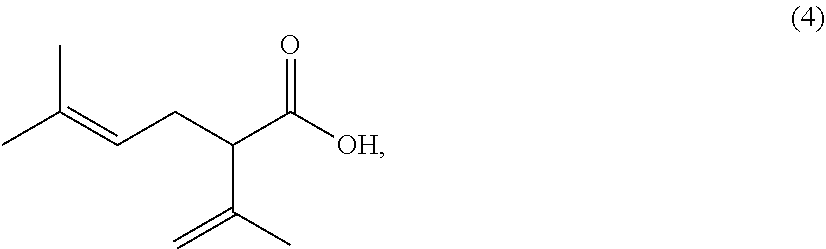
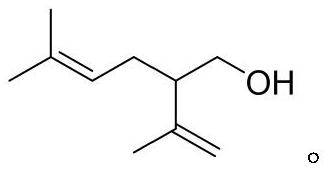
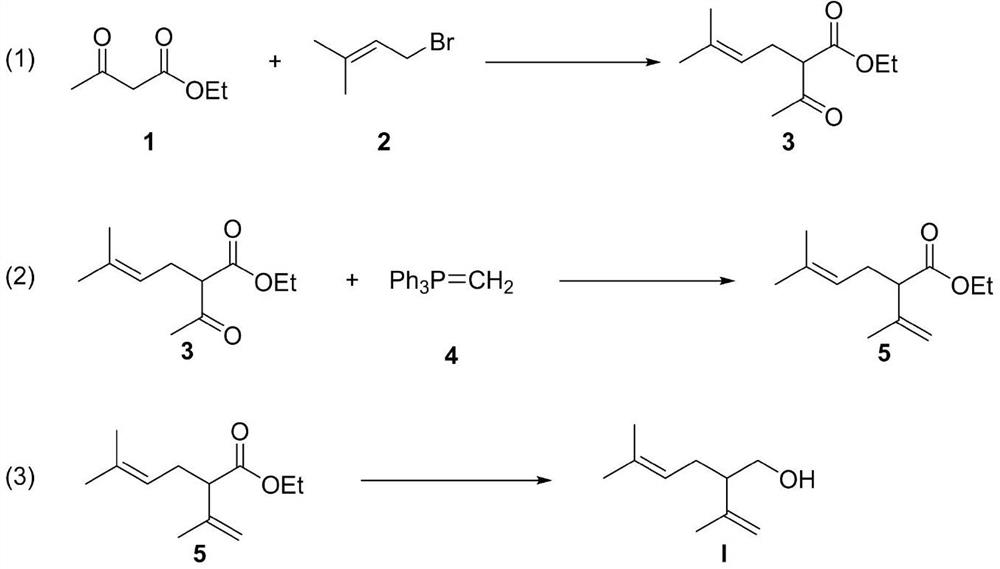
Processes for preparing 2-isopropenyl-5-methyl-4-hexenoic acid, 2-isopropenyl-5-methyl-4-hexen-1-ol, and a carboxylate ester thereof
ActiveUS11286227B2Suppressing eliminating decreaseQuality improvementGroup 4/14 element organic compoundsPreparation from carboxylic acid halidesHexacosenoic acidGrignard reagent
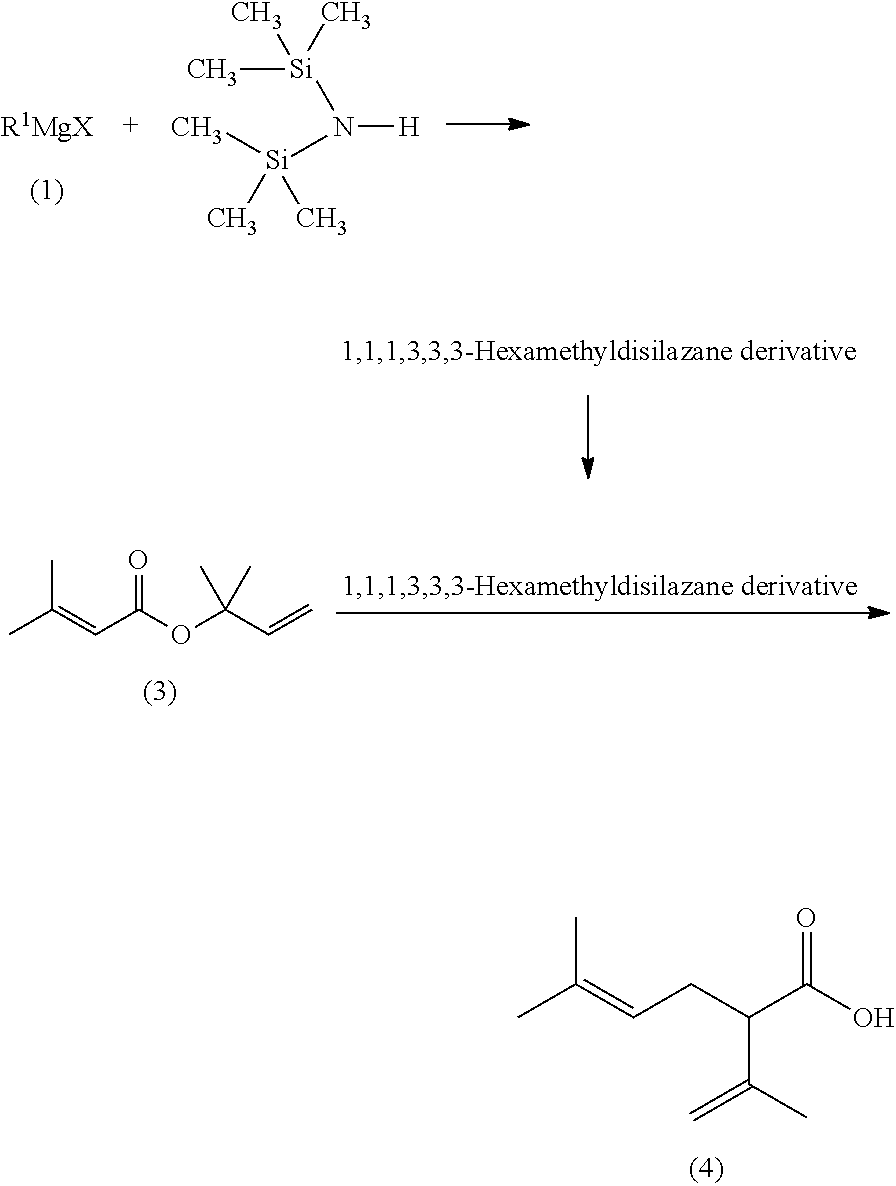
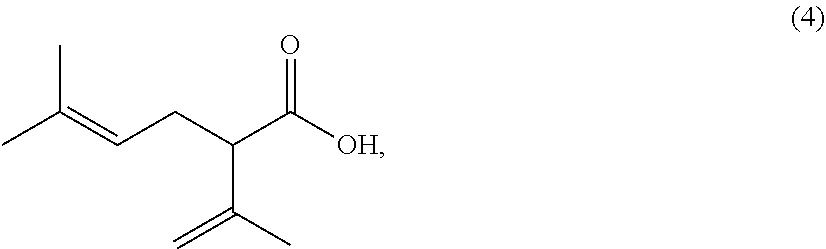
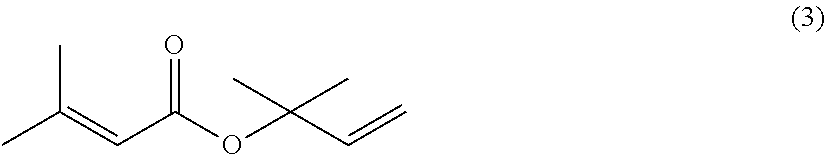
The present invention provides a process for preparing 2-isopropenyl-5-methyl-4-hexenoic acid of the following formula (4), comprising steps of:subjecting a Grignard reagent of the following general formula (1), wherein R1 represents a linear, branched, or aromatic monovalent hydrocarbon group having 1 to 8 carbon atoms, and X represents a chlorine atom, a bromine atom, or an iodine atom, and 1,1,1,3,3,3-hexamethyldisilazane to a deprotonation reaction to form a 1,1,1,3,3,3-hexamethyldisilazane derivative; and subjecting 2-methyl-3-buten-2-yl 3-methyl-2-butenoate of the following formula (3) to a rearrangement reaction in the presence of the 1,1,1,3,3,3-hexamethyldisilazane derivative to form 2-isopropenyl-5-methyl-4-hexenoic acid (4).
Owner:SHIN ETSU CHEM IND CO LTD
Aminohexenoic acid intermediate and preparation method thereof
PendingCN113754538AEasy to removeEfficient removalOrganic compound preparationCarboxylic acid esters preparationHexacosenoic acidButene
The invention provides an aminohexenoic acid intermediate 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate and a preparation method thereof, application of the aminohexenoic acid intermediate 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate in preparation of aminohexenoic acid, an aminohexenoic acid product and a preparation method thereof. The preparation method of the 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate comprises the steps: (1) reacting 1, 4-dichloro-2-butene with diethyl malonate in a sodium ethoxide solution to obtain a reaction solution containing 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate; and (2) carrying out purification treatment on the reaction solution, wherein the purification treatment comprises the steps: directly cooling and filtering the reaction solution, and collecting a filtrate containing 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate. The 2-vinyl cyclopropane-1, 1-diethyl dicarboxylate prepared by the preparation method is high in yield, high in purity, few in solvent residue and raw material residue, simple, convenient and rapid to operate, free of wastewater discharge and suitable for large-scale production.
Owner:WUHAN WUYAO SCI & TECH
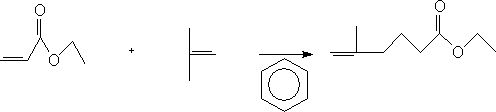
A kind of preparation method of ethyl 5-methyl-5-hexenoate
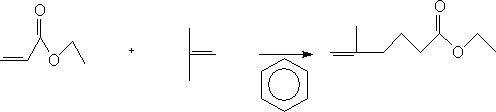
ActiveCN112062676BEasy to operateLow costPhysical/chemical process catalystsOrganic compound preparationSodium bicarbonateHexacosenoic acid
The invention discloses a preparation method of ethyl 5-methyl-5-hexenoate, comprising the following steps: 1) adding ethyl acrylate, benzene and a catalyst into a four-necked flask, controlling the temperature to 25-30° C. Add isobutylene, keep the temperature for 24-60h, add aqueous sodium bicarbonate solution, stir for 0.5-1h, then add a mixture of hydrochloric acid and water, stir for 0.5h, stand still for 0.5h, separate layers, and wash with water to obtain an aqueous layer and a benzene layer; 2 ) adding the benzene layer into the barrel, adding a dehydrating agent for dehydration, concentrating, first at normal pressure, then under reduced pressure, rectifying, and collecting the normal boiling fraction to obtain 5-methyl-5-ethyl hexenoate. The invention provides a preparation method of ethyl 5-methyl-5-hexenoate, using aluminum trichloride and tungsten hexachloride synergistically as a catalyst, and the total yield can reach more than 46.7%. When butanol is used as a dehydrating agent, the product purity can reach more than 97.5%; the preparation method of the invention is simple in operation and low in cost.
Owner:太仓市茜泾化工有限公司
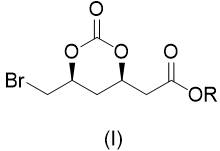
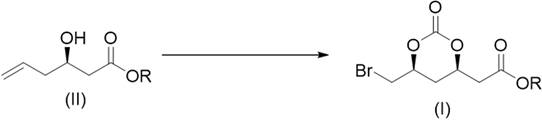
Preparation method of 2-((4r,6s)6 bromomethyl 2-oxo-1,3-dioxane-4-yl)acetate
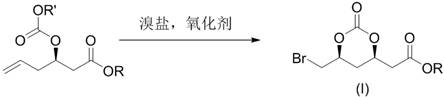
ActiveCN107188880BRaw materials are easy to getSave raw materialsCarboxylic acid ester formation/introductionHexacosenoic acidHypochlorite
The present disclosure belongs to the technical field of organic synthesis and particularly relates to a preparation method for 2-((4R,6S)-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate. The 2-((4R,6S)-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate is a key chiral intermediate for preparation of statin antilipemic agents. In the present disclosure, the 2-((4R,6S)-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate is obtained by bromination and cyclization of 3-((substituted oxycarbonyl)oxy)-5-hexenoate as raw material with hypochlorite and bromide in an organic solvent in the presence of CO2. The method of the present disclosure has the advantages of readily available raw material, mild reaction conditions, easy operation, low cost, excellent atomic economy and less by-products, and is applicable to industrial production.
Owner:FUDAN UNIV
Omega-3 compositions and methods relating thereto
ActiveUS11241408B2Improve abilitiesSmall sizeOrganic active ingredientsEmulsion deliveryHexacosenoic acidDHA - Docosahexaenoic acid
Owner:PHARMAKO BIOTECH PTY LTD
Preparation method of 5-methyl-5-ethyl hexenoate
ActiveCN112062676AEasy to operateLow costPhysical/chemical process catalystsOrganic compound preparationSodium bicarbonateHexacosenoic acid
The invention discloses a preparation method of 5-methyl-5-ethyl hexenoate. The method comprises the following steps: 1) adding ethyl acrylate, benzene and a catalyst into a four-neck flask, controlling the temperature to be 25-30 DEG C, introducing isobutene, preserving heat for 24-60 hours, adding a sodium bicarbonate aqueous solution, stirring for 0.5-1 hour, adding a mixed solution of hydrochloric acid and water, stirring for 0.5 hour, standing for 0.5 hour, layering, and washing with water to obtain a water layer and a benzene layer; and 2) adding the benzene layer into a barrel, adding adehydrating agent, dehydrating, concentrating, carrying out normal pressure distillation and reduced pressure distillation in sequence, and collecting the azeotropic fraction to obtain the 5-methyl-5-ethyl hexenoate. According to the preparation method of the 5-methyl-5-ethyl hexenoate, aluminum trichloride and tungsten hexachloride are synergistically used as catalysts, the total yield can reach46.7% or above, anhydrous sodium sulfate and n-butyl alcohol are used as dehydrating agents, and the product purity can reach 97.5% or above; and the preparation method disclosed by the invention issimple to operate and relatively low in cost.
Owner:太仓市茜泾化工有限公司
Processes for preparing 2-isopropenyl-5-methyl-4-hexenoic acid, 2-isopropenyl-5-methyl-4-hexen-1-ol, and a carboxylate ester thereof
ActiveCN113527079AEfficient preparationNo reduction in conversion rateGroup 4/14 element organic compoundsPreparation from carboxylic acid halidesHexacosenoic acidGrignard reagent
The present invention provides a process for preparing 2-isopropenyl-5-methyl-4-hexenoic acid of the following formula (4), comprising steps of:subjecting a Grignard reagent of the following general formula (1), wherein R<1> represents a linear, branched, or aromatic monovalent hydrocarbon group having 1 to 8 carbon atoms, and X represents a chlorine atom, a bromine atom, or an iodine atom, and 1,1,1,3,3,3-hexamethyldisilazane to a deprotonation reaction to form a 1,1,1,3,3,3-hexamethyldisilazane derivative; and subjecting 2-methyl-3-buten-2-yl 3-methyl-2-butenoate of the following formula (3) to a rearrangement reaction in the presence of the 1,1,1,3,3,3-hexamethyldisilazane derivative to form 2-isopropenyl-5-methyl-4-hexenoic acid (4).
Owner:SHIN ETSU CHEM CO LTD
Synthetic method and application of 4-oxo-trans-2-hexenal analogues
ActiveCN112094181BHigh trapping activityImprove stabilityPreparation by organometalhalide reactionBiocideHexacosenoic acidChemical compound
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
A method for preparing (r)-3-hydroxy-5-hexenoate
ActiveCN107119081BSimple and fast operationMild reaction conditionsOrganic compound preparationComponent separationHexacosenoic acidBiochemical engineering
Owner:FUDAN UNIV
Processes for preparing 2-isopropenyl-5-methyl-4-hexenoic acid, 2-isopropenyl-5-methyl-4-hexen-1-ol, and a carboxylate ester thereof
ActiveUS20210323902A1Increase conversionsHigh purityGroup 4/14 element organic compoundsPreparation from carboxylic acid halidesHexacosenoic acidProtonation
The present invention provides a process for preparing 2-isopropenyl-5-methyl-4-hexenoic acid of the following formula (4), comprising steps of:subjecting a Grignard reagent of the following general formula (1), wherein R1 represents a linear, branched, or aromatic monovalent hydrocarbon group having 1 to 8 carbon atoms, and X represents a chlorine atom, a bromine atom, or an iodine atom, and 1,1,1,3,3,3-hexamethyldisilazane to a deprotonation reaction to form a 1,1,1,3,3,3-hexamethyldisilazane derivative; and subjecting 2-methyl-3-buten-2-yl 3-methyl-2-butenoate of the following formula (3) to a rearrangement reaction in the presence of the 1, 1, 1,3,3,3-hexamethyldisilazane derivative to form 2-isopropenyl-5-methyl-4-hexenoic acid (4).
Owner:SHIN ETSU CHEM IND CO LTD
Synthesis method of (+/-)-lavandulol
PendingCN114380661AReduce pollutionHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationHexacosenoic acidOrganosolv
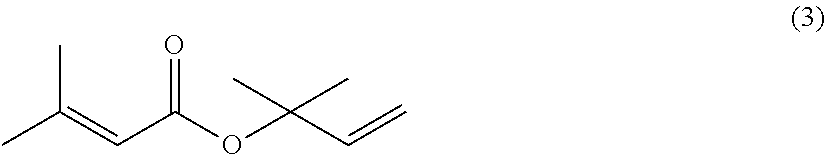
The invention discloses a synthesis method of (+ / -)-lavandulol. The synthesis method comprises the following steps: (1) carrying out substitution reaction on ethyl acetoacetate and 1-bromo-3-methyl-2-butene under an alkaline condition to prepare 2-acetyl-5-methylhex-4-olefine acid ethyl ester; (2) carrying out a Wittig reaction on the ethyl 2-acetyl-5-methylhexyl-4-olefine acid ester and a methyl Wittig reagent in an organic solvent, so as to prepare ethyl lavandulamate; and (3) reducing the ester group of the ethyl lavandulate into alcohol under the action of lithium aluminum hydride to prepare the final product (+ / -)-lavandulol. The method has the advantages of low raw material price, short process route, few post-treatment steps and high reaction yield, and is a production process with a relatively good production prospect.
Owner:CHINA TOBACCO ANHUI IND CO LTD
Liriomyza sativae attractant and controlled release system and application thereof
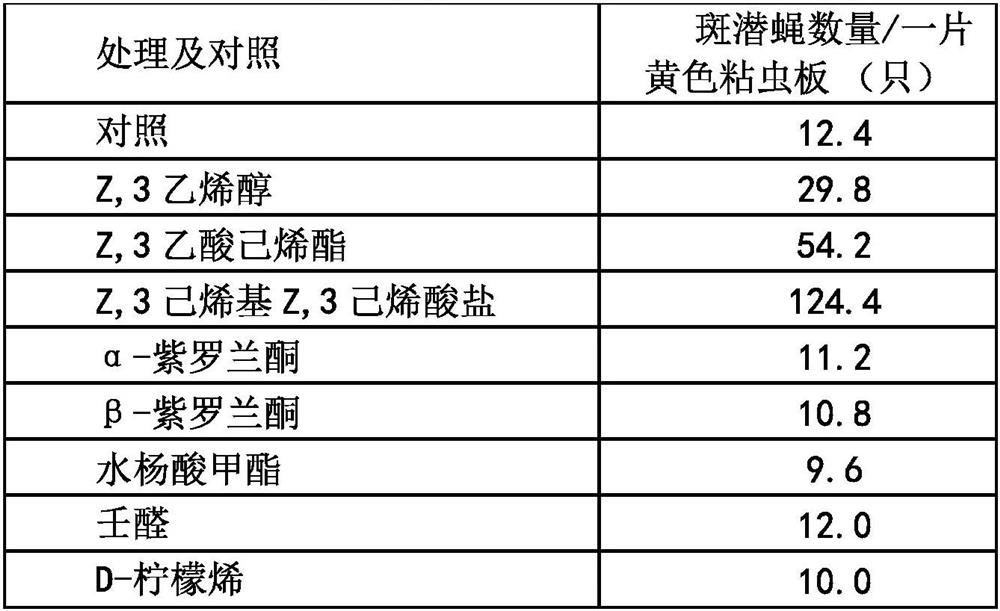
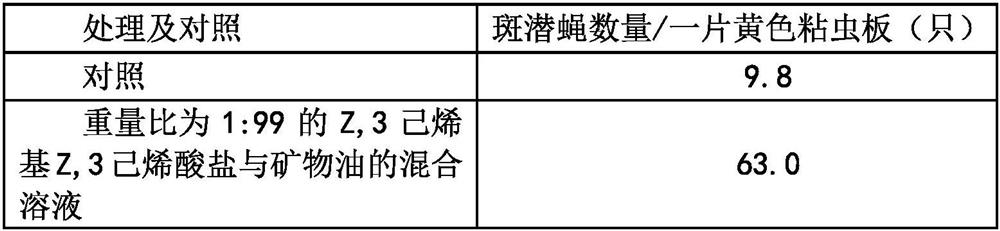
PendingCN114847287AAccurately lure and disinfectReduce in quantityBiocidePest attractantsHexacosenoic acidVermin
Owner:NANJING SINO GREEN BIOTECH
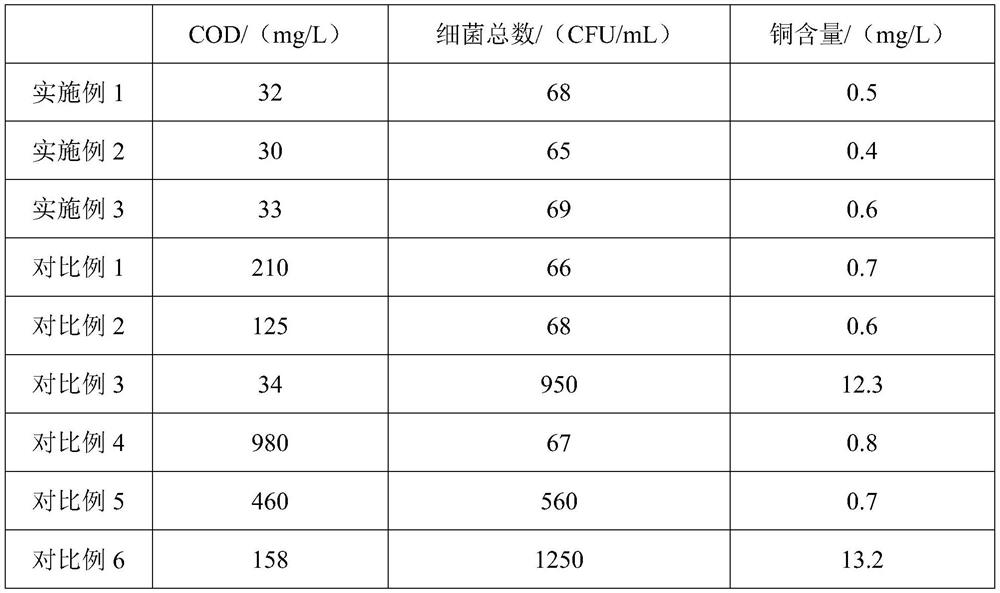
High-concentration organic wastewater treatment process
ActiveCN114409194AImprove conductivityEasy to stretchWater contaminantsUltrafiltrationHexacosenoic acidUltrafiltration
The invention discloses a high-concentration organic wastewater treatment process, and relates to the technical field of wastewater treatment. The high-concentration organic wastewater treatment process comprises primary degradation, secondary degradation, tertiary degradation and the like. The primary degradation pool and the secondary degradation pool are separated by using a Nafion membrane, one end of an ultrafiltration membrane of the tertiary degradation pool is connected with a carbon nanotube filter body of the primary degradation pool, and the other end of the ultrafiltration membrane of the tertiary degradation pool is connected with a carbon nanotube filter body of the secondary degradation pool by using a lead; in the primary degradation, a carbon nanotube filter body is used for assisting anaerobic bacteria degradation, in the secondary degradation, aeration is used for assisting the carbon nanotube filter body degradation, and in the third degradation, an ultrafiltration membrane is used for degradation; the carbon nano tube filter body is prepared from dipentanedione isopentyl glycol, lactic acid and a functionalized carbon nano tube; the ultrafiltration membrane is prepared by modifying poly (3-amino-4-carbonyl hexenoic acid) with bis (3-carbonyl ethyl butyrate) diformaldoxy silane, and the ultrafiltration membrane is prepared from poly (3-amino-4-carbonyl hexenoic acid); the high-concentration organic wastewater treatment process is high in bactericidal activity, and the COD value and the heavy metal ion content of the treated organic wastewater are relatively low.
Owner:JIANGSU WATER BUSINESS DOCTOR ENVIRONMENTAL PROTECTION TECH
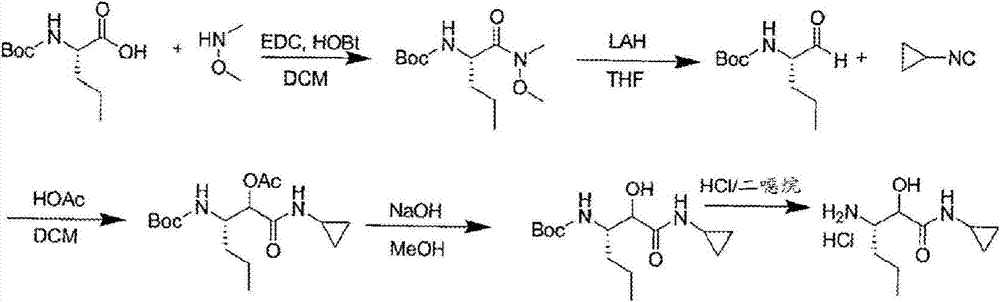
A method for the preparation of 3-amino-n-cyclopropyl-2-hydroxyl-hexanamide
InactiveCN104812733ALow toxicityToxicOrganic compound preparationCarboxylic acid amides preparationAminalNitrile
The present invention discloses a method for preparing 3-amino-N-cyclopropyl-2-hydroxyl-hexanamide. The invention relates to the technical field of the preparation of pharmaceutical intermediates. The method uses trans-2-hexenoic acid as the starting material, through the steps of epoxidation, ring-opening by nitrile, amidation, etc., to obtain the final product 3-amino-N-cyclopropyl-2-hydroxyl-hexanamide. The method uses easily-obtainable materials, requires mild reactive conditions, and adopts stable processes. Therefore it is suitable for large scale industrial production.
Owner:JANSSEN PHARMA NV
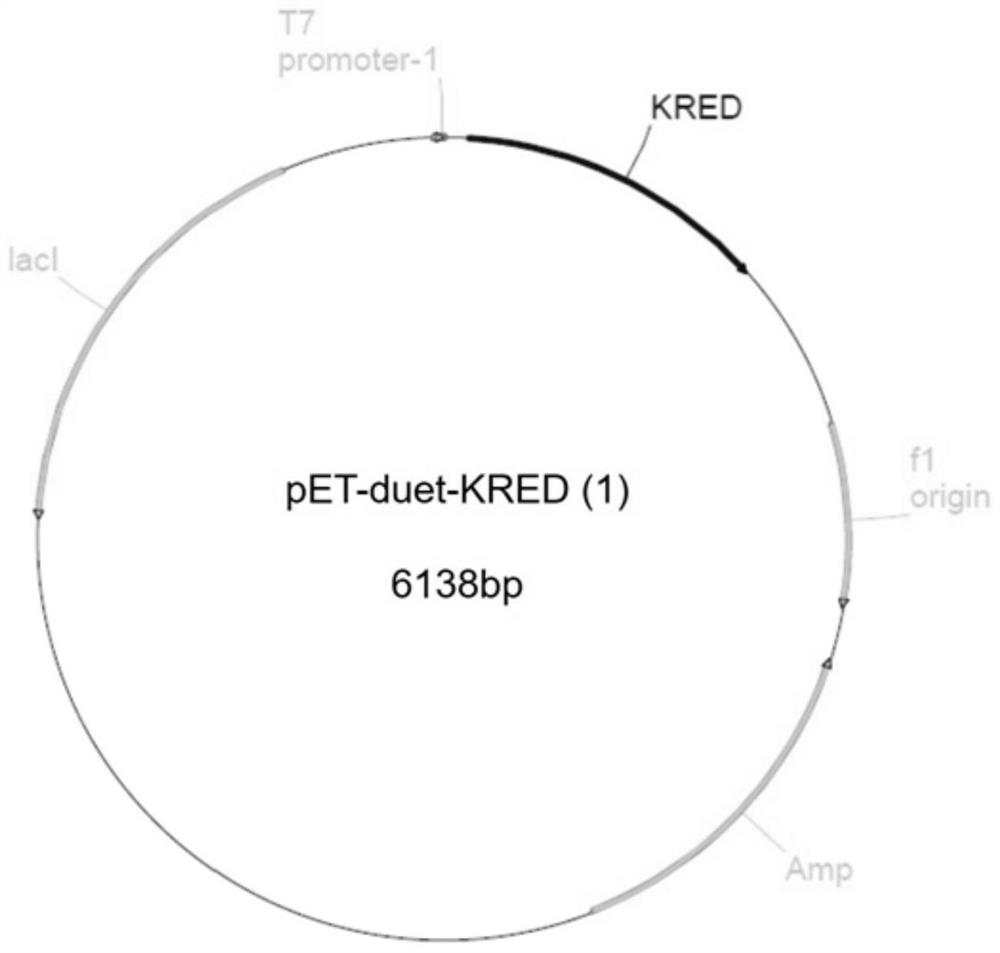
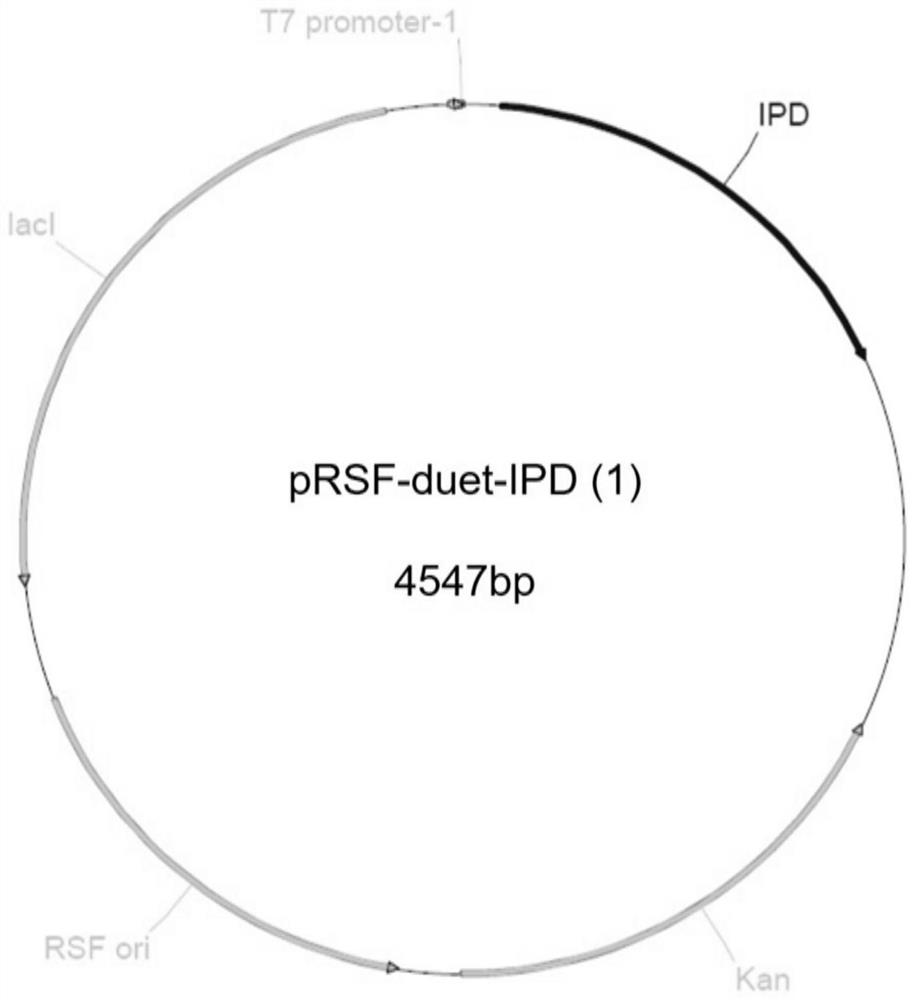
A kind of engineering bacteria and its application in the preparation of (r)-3-hydroxyl-5-hexenoate
ActiveCN108359626BSimple and fast operationImprove efficiencyBacteriaMicroorganism based processesHexacosenoic acidBase J
The invention belongs to the technical field of biopharmaceuticals, and specifically discloses an engineering bacterium and its preparation ( R )-3-hydroxy-5-hexenoate (I). The engineering bacterium of the present invention includes a host cell and two target genes co-transformed into the host cell, which are respectively the ketoreductase gene shown in SEQ ID NO.1 and the isopropyl gene shown in SEQ ID NO.2. Alcohol dehydrogenase gene. In the present invention, the ketoreductase gene shown in SEQ ID NO.1 and the isopropanol dehydrogenase gene shown in SEQ ID NO.2 are jointly introduced into the host cell to construct engineering bacteria that can express ketoreductase KRED and isopropanol dehydrogenase Hydrogenase IPD, through the catalysis of KRED and the recycling of IPD to the coenzyme NADPH, realizes the efficient and high stereoselective reduction of 3-carbonyl-5-hexenoic acid ester (II) ( R )‑3‑hydroxy‑5‑hexenoate (I).
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com