Analysis method of bacitracin and components thereof
An analysis method, the technology of bacitracin, which is applied in the field of analysis of bacitracin and its components, can solve the problems of being unsuitable for mass spectrometry analysis and unable to realize online determination of unknown components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
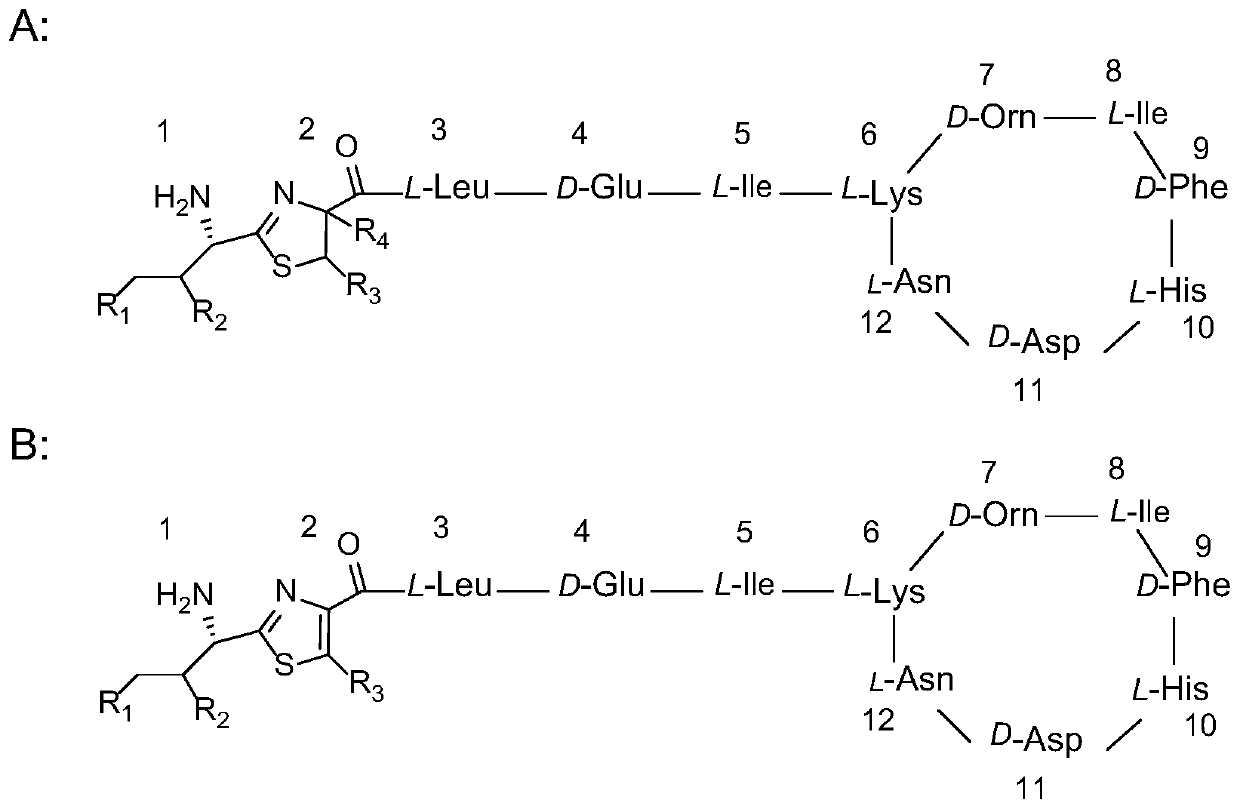
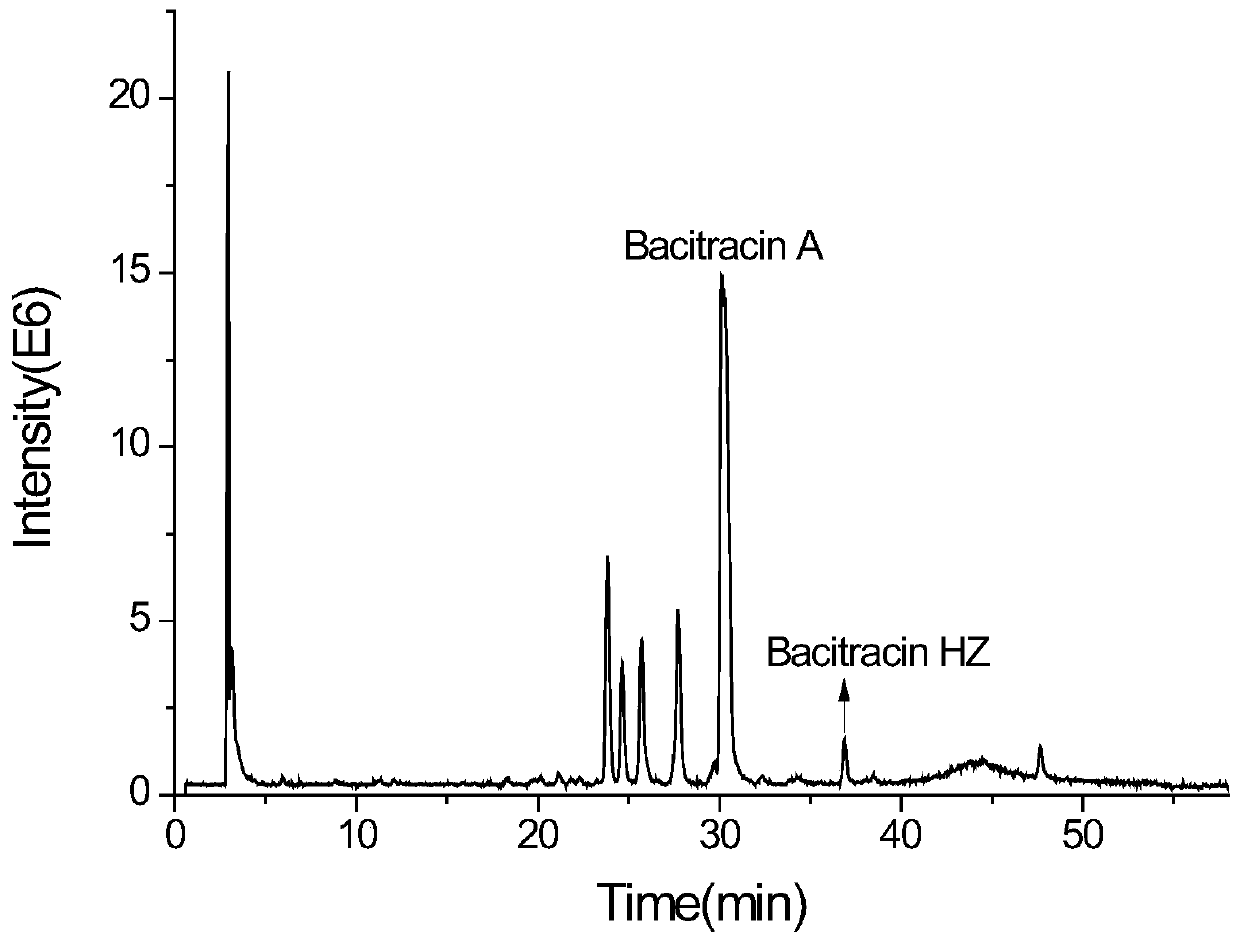
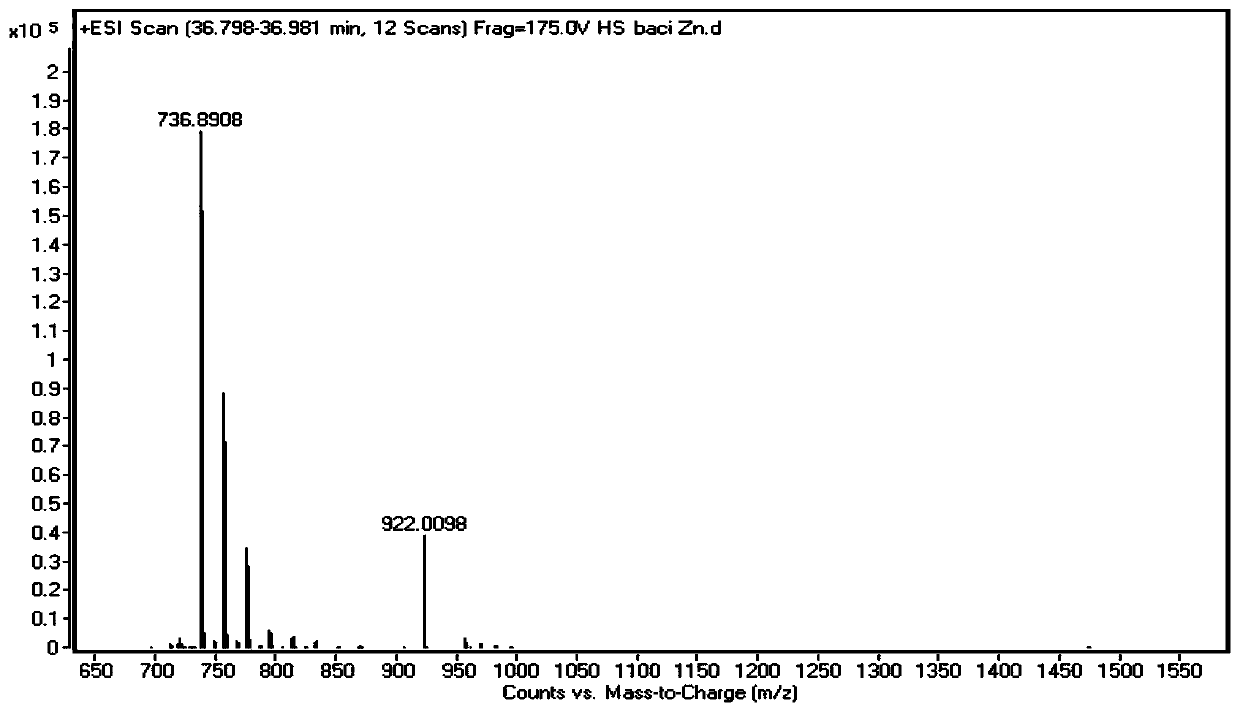
[0055] This embodiment provides a new bacitracin named bacitracin HZ (bacitracin HZ). The components of the bacitracin are double-methylated and vinylated bacitracin A or double-methylated and ethylated bacitracin Y. Among them, the constituent units of methyleneized and vinylated bacitracin A include 1-position isoleucine, 2-position cysteine, 3-position leucine, 4-position glutamic acid, 5-position isoleucine , 6-lysine, 7-ornithine, 8-position isoleucine, 9-position phenylalanine, 10-position histidine, 11-position aspartic acid, 12-position asparagine, 1-position isoleucine Amino acid and 2-cysteine form a thiazoline ring, and 6-12 amino acids form a cyclic 7-peptide. The constituent units of double-methylated and ethylated bacitracin Y include 1-position isoleucine, 2-position cysteine, 3-position leucine, 4-position glutamic acid, 5-position isoleucine, 6-lysine, 7-ornithine, 8-position isoleucine, 9-position phenylalanine, 10-position histidine, 11-position aspartic...
Embodiment 2
[0062] This example provides the application of Bacitracin HZ in medicine. A bacitracin bulk drug or pharmaceutical combination comprising bacitracin HZ and a pharmaceutical preparation comprising the aforementioned bulk drug or pharmaceutical composition.
[0063] The composition of the pharmaceutical preparation comprises bacitracin for injection, bacitracin ointment and bacitracin eye ointment.
[0064] Wherein, the specification of the injection is 1000 units per milliliter (dissolved in isotonic sodium chloride injection);
[0065] The specifications of the ointment are 1g: 500 units, 5g: 4000 units;
[0066] The specification of eye ointment is 1g: 500 units, 2g: 1000 units.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com