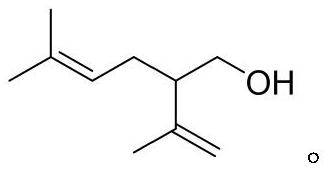
Synthesis method of (+/-)-lavandulol
A synthetic method, the technology of lavandulol, which is applied in the field of organic synthesis, can solve the problems of incapability of large-scale industrial production, limitation of application of lavandulol, cumbersome synthesis steps, etc., and achieve the effects of convenient industrial production, low production cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
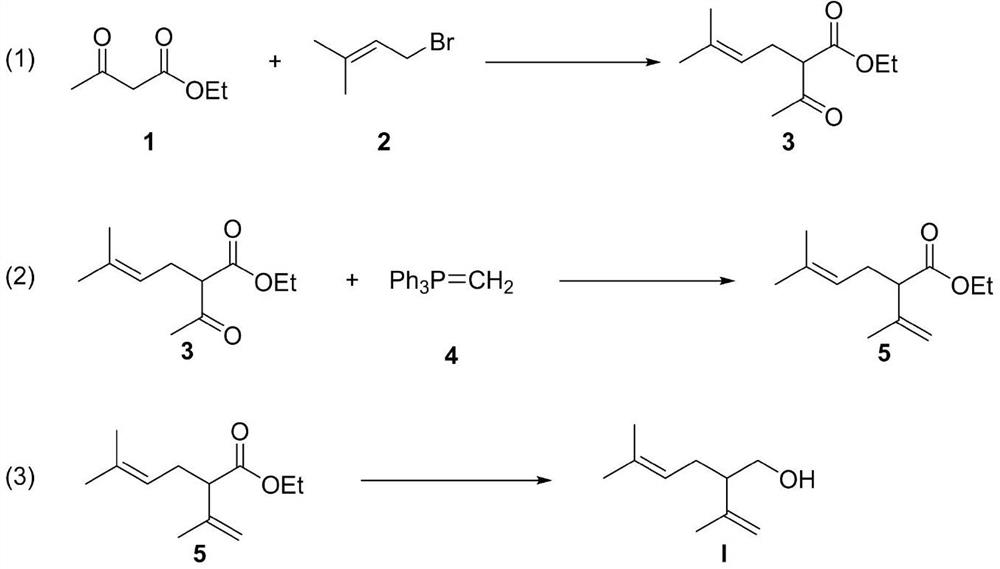
[0021] Example 1: Synthesis of ethyl 2-acetyl-5-methylhex-4-enoate
[0022] Add ethyl acetoacetate (50mmol), 1-bromo-3-methyl-2-butene (60mmol), potassium carbonate (75mmol) and potassium iodide (40mmol) into a 250mL reaction flask in sequence, add N,N-dimethyl Dimethyl formamide (100 mL) and started stirring to make it evenly stirred. The reaction was stirred at room temperature for 24 hours. After the raw materials disappeared completely, the stirring was stopped. The reaction mixture was washed successively with water (100 mL×3) and saturated brine (100 mL), and the organic phases were combined and dried over anhydrous magnesium sulfate (5 g). The insoluble matter was removed by filtration, and the excess organic solvent was removed by concentration under reduced pressure to obtain ethyl 2-acetyl-5-methylhex-4-enoate, which was directly used in the next reaction without purification.
Embodiment 2
[0023] Embodiment 2: the synthesis of ethyl lavender
[0024] In a 250 mL round bottom flask, add 2-acetyl-5-methylhex-4-enoic acid ethyl ester 3 and methyl Wittig reagent (50 mmol) successively, and add toluene (100 mL) to dissolve, and the reaction is stirred at room temperature for 72 hours . After the reaction was completed, diethyl ether (100 mL) was added, insoluble impurities were removed by filtration, excess organic solvent was removed by concentration under reduced pressure, and the crude product was purified by column chromatography (the eluent volume ratio was petroleum ether:ethyl acetate=50:1), Ethyl lavender (6.08 g, yield 62%) was obtained.
Embodiment 3
[0025] Embodiment 3: the synthesis of lavandulol
[0026] In a 250mL round-bottomed flask, add ethyl lavender (31mmol) obtained in the previous step, add ether (100mL) to dissolve, cool the reaction mixture to 0°C, and add lithium aluminum hydride (93mmol) batch by batch to the reaction. The reaction was slowly warmed to room temperature and stirred at room temperature for 6 hours. After the reaction is completed, add sodium sulfate decahydrate (NaSO 4 10H 2 (2, 300mmol) quenched the reaction, filtered to remove insoluble impurities, and the filter cake was washed with ether (100mL), the organic phases were combined and dried with anhydrous magnesium sulfate (10g), concentrated under reduced pressure to remove excess organic solvent, and the crude product was purified by column chromatography (The volume ratio of the eluent is petroleum ether:ethyl acetate=3:1) to obtain lavandulol (4.39 g, yield 92%).
[0027] 1 H NMR (600MHz, CDCl 3 )δ5.03(tt, J=7.9, 1.3Hz, 1H), 4.91-4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com