A kind of engineering bacteria and its application in the preparation of (r)-3-hydroxyl-5-hexenoate
A technology of hexenoate and engineering bacteria is applied in the application field of engineering bacteria to prepare -3-hydroxy-5-hexenoate, which can solve the problems of cumbersome production operations, increase industrial production costs and the like, and achieve simple and convenient operation. , the effect of low cost and excellent practical industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
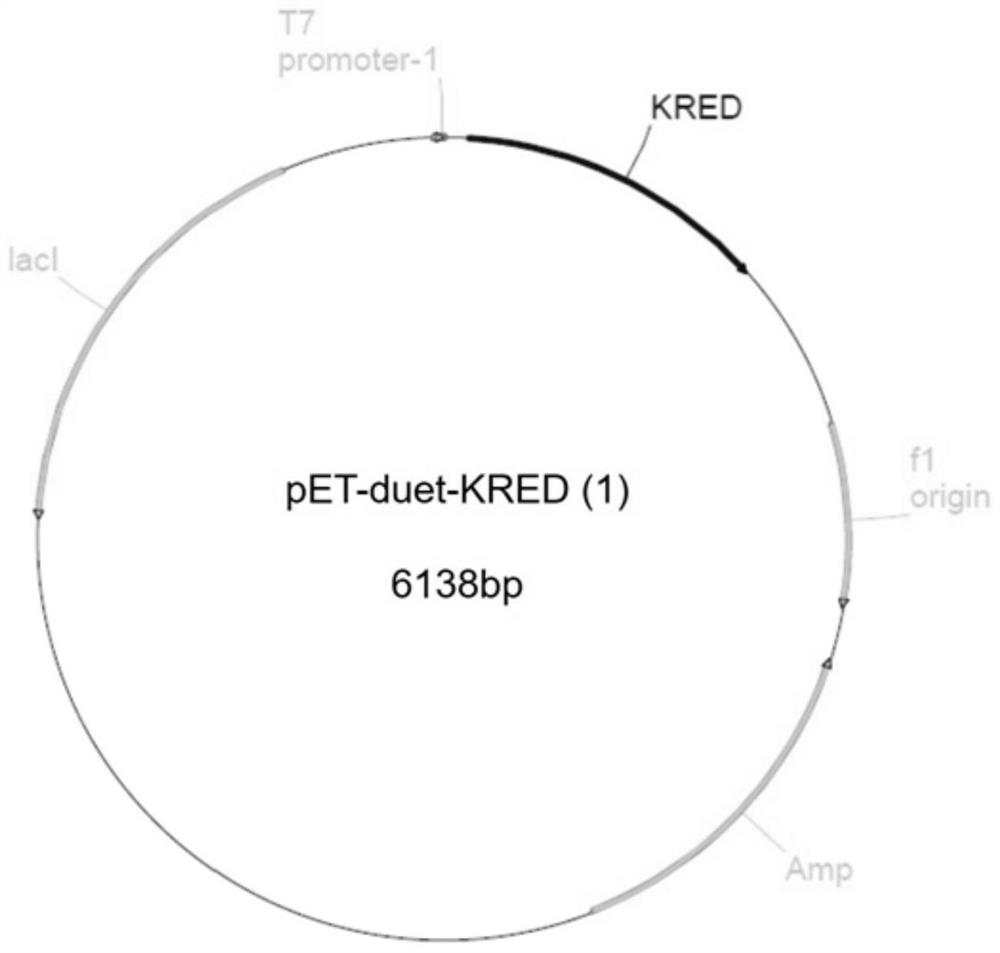
[0030] Embodiment 1, the preparation of recombinant plasmid pET-duet-KRED (1)
[0031] Using the recombinant plasmid pET-24b-KRED previously reported (Chinese patent application CN107119081A) as a template, the ketoreductase (KRED) gene was cloned with primers F_KRED / R_KRED to obtain a 759bp KRED gene (SEQ ID NO.1).
[0032] The sequence of primer F_KRED is:
[0033] 5'-TTTAACTTTAAGAAGGAGATATACCATGACCGACCGTCTGAAAGGTAAAG (SEQ ID NO.3)
[0034] The sequence of primer R_KRED is:
[0035] 5'-CTGCAGGCGCGCCGAGCTCGAATTCTCACTGCGCGGTCCAGCCGCCAT (SEQ ID NO.4)
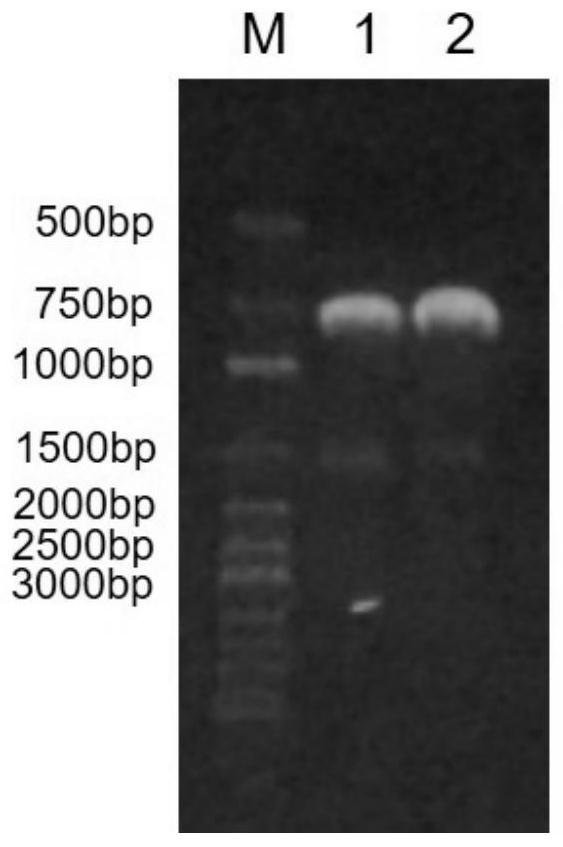
[0036] The gene fragment containing the ketoreductase gene was recombined with the double digestion product (NcoI and EcoRI) of the pET-duet plasmid, and transformed into the cloning host E.coli DH5. Use primers F_KRED / R_KRED for colony PCR verification, transform recombinants, extract recombinant plasmids, and perform sequencing. The recombinant plasmid with correct sequencing results is the recombinant plasmid pET-duet-KRED...
Embodiment 2
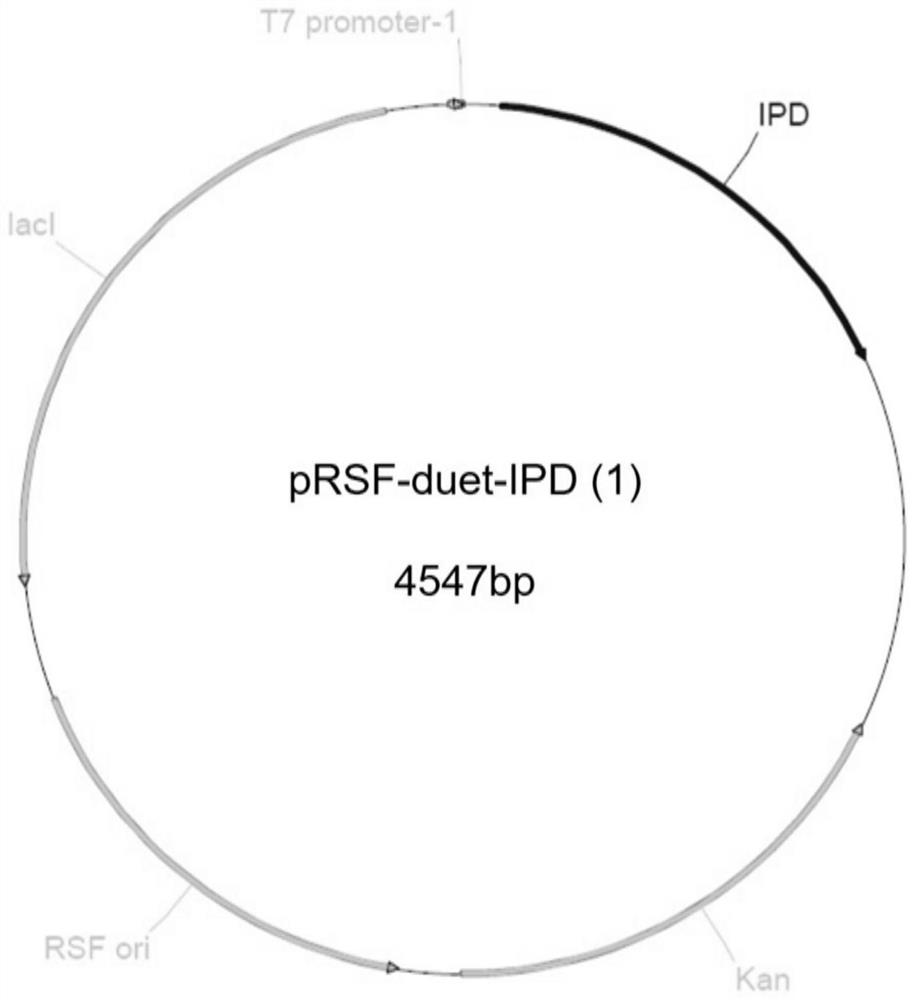
[0037] Embodiment 2, preparation of recombinant plasmid pRSF-duet-IPD (1)
[0038] Using the recombinant plasmid pET-22b-IPD previously reported (Chinese patent application CN107119081A) as a template, the isopropanol dehydrogenase (IPD) gene was cloned with primers F_IPD / R_IPD to obtain a 759bp IPD gene (SEQ ID NO.2) . The sequence of primer F_IPD is:
[0039] 5'-TTTAACTTTAATAAGGAGATATACCATGACTGATCGTTTAAAAGGCAAAGT (SEQ ID NO.5)
[0040] The sequence of primer R_IPD is:
[0041] 5'-CTGCAGGCGCGCCGAGCTCGAATTCTTATTGAGCAGTGTATCCACCAT (SEQ ID NO. 6)
[0042] The gene fragment containing the isopropanol dehydrogenase gene was recombined with the double digestion product (NcoI and EcoRI) of the pRSF-duet plasmid, and transformed into the cloning host E.coli DH5. Use primers F_IPD / R_IPD for colony PCR verification, transform recombinants, extract recombinant plasmids, and perform sequencing. The recombinant plasmid with correct sequencing results is the recombinant plasmid pRSF-d...
Embodiment 3
[0043] Embodiment 3, construction and induced expression of genetically engineered bacteria
[0044] Use the plasmids pET-duet-KRED(1) and pRSF-duet-IPD(1) constructed in Examples 1 and 2 to co-transform the expression host E.coli JM109(DE3), screen to obtain positive clones, and name the engineered bacteria For Eco-KRED-IPD.
[0045]The engineered bacteria were inoculated into 5 mL liquid LB medium containing kanamycin and ampicillin for activation for 8 hours (37° C., 180 rpm). The above-mentioned activated culture was transferred to 50 mL liquid LB medium containing kanamycin and ampicillin at an inoculum size of 1 / 100 and cultured overnight (37° C., 180 rpm). Take the overnight culture and transfer it to 5L liquid medium containing kanamycin (50mg) and ampicillin (50mg) with an inoculum size of 1 / 100 (placed in a 7L fermenter, containing 2% tryptone, 1% yeast powder, 1% NaCl) fermentation culture (30°C, 300rpm) to OD 600 When it reached 10, IPTG (70 mg) was added, and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com