A method for preparing (r)-3-hydroxy-5-hexenoate
A technology of hexenoic acid ester and hydroxyl group, which is applied in the field of preparation of (R)-3-hydroxy-5-hexenoic acid ester, can solve the problems of poor stereoselectivity and unsuitability for industrial production, and achieve easy operation and good practicality Industrial application value, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
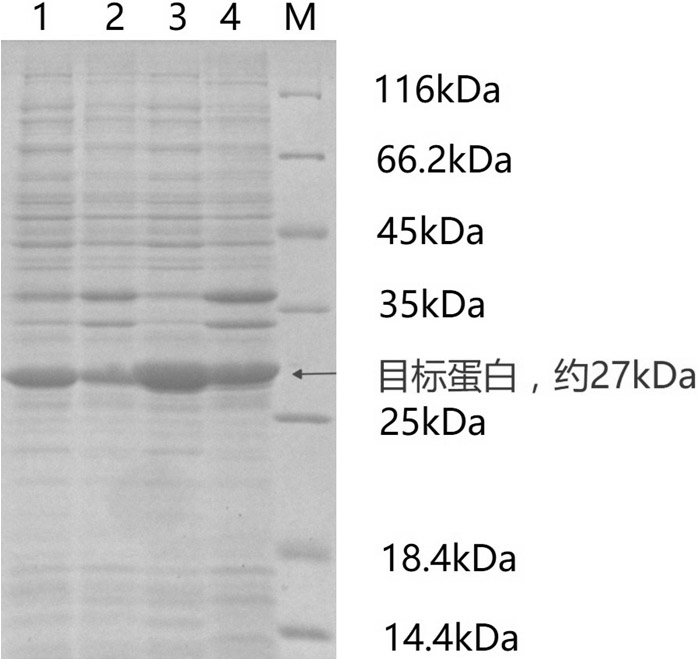
[0035] Embodiment 1, the fermenter production process of ketoreductase crude enzyme
[0036] Ligate the gene fragment containing the ketoreductase gene with the digested product of the pET-24b plasmid, and transform it into competent E. coli E. coli In the JM109(DE3) strain, positive clones were screened and inoculated into 5 mL liquid LB medium containing kanamycin for activation for 8 hours (37°C, 180rpm).
[0037] Take the above-mentioned activated culture and transfer it to 50 mL liquid LB medium containing kanamycin at an inoculum size of 1 / 100 and culture overnight (37°C, 180rpm).
[0038] Take the overnight culture and transfer it to 5L liquid medium containing kanamycin (100mg) at an inoculum size of 1 / 100 (placed in a 7L fermenter, containing 2% tryptone, 1% yeast powder, 1 %NaCl) fermentation culture (30°C, 300rpm) to OD 600 When it reaches 10, add IPTG (50mg) and culture overnight at 25°C (glycerol is added during the process, and the pH is controlled at 6.8). C...
Embodiment 2
[0039] Embodiment 2, the fermenter production process of isopropanol dehydrogenase
[0040] Ligate the gene fragment containing the isopropanol dehydrogenase gene with the digested product of the pET-22b plasmid, and transfer it into competent Escherichia coli E. coli In the JM109(DE3) strain, positive clones were screened and inoculated into 5 mL of liquid LB medium containing ampicillin for activation for 8 hours (37°C, 180rpm).
[0041] Take the above-mentioned activated culture and transfer it to 50 mL liquid LB medium containing ampicillin at an inoculum size of 1 / 100 and culture overnight (37°C, 180rpm).
[0042] Take the overnight culture and transfer it to 5L liquid medium containing ampicillin (100mg) at an inoculum size of 1 / 100 (placed in a 7L fermenter, containing 2% tryptone, 1% yeast powder, 1% NaCl ) fermentation culture (30°C, 300rpm) to OD 600 When it reaches 10, add IPTG (50mg) and culture overnight at 25°C (glycerol is added during the process, and the pH...
Embodiment 3
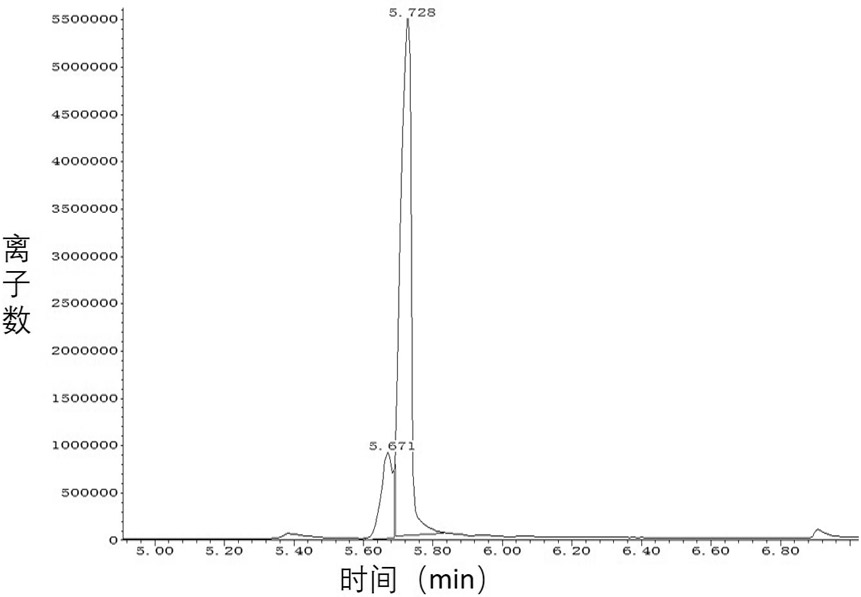
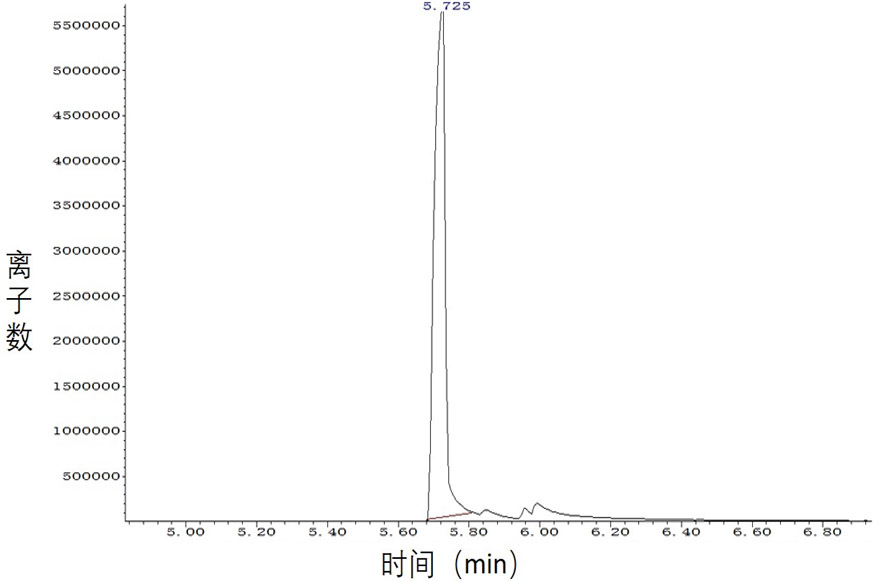
[0043] Embodiment 3, ketoreductase catalyzes asymmetric reduction generation ( R )-3-Hydroxy-5-hexenoic acid methyl ester (100g grade)
[0044] Add 0.5L of phosphate buffer (100mM, pH7.5) and 0.1L of isopropanol to a 2L reaction bottle, mix well and add 0.2L of the crude ketoreductase solution obtained in Example 1 and 0.1L of the ketoreductase solution obtained in Example 2 The obtained isopropanol dehydrogenase crude enzyme solution, then add 5mg coenzyme NADP + , and finally 100 g of the substrate 3-carbonyl-5-hexenoic acid methyl ester was added. The reaction was carried out at 30°C and monitored by GC-MS. After 16 hours of reaction, the conversion rate of the product was greater than 99%, and the reaction was terminated.
[0045] Silica gel was added to the reaction solution, stirred for 15 min, filtered, the filter cake was rinsed with ethyl acetate, the filtrate was separated into layers, and the aqueous layer was extracted twice with ethyl acetate. The organic layers ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com