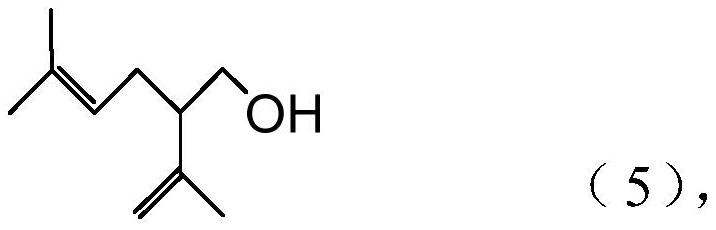
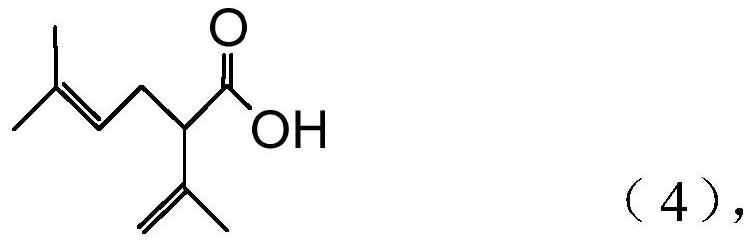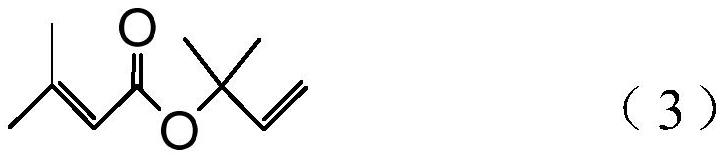Processes for preparing 2-isopropenyl-5-methyl-4-hexenoic acid, 2-isopropenyl-5-methyl-4-hexen-1-ol, and a carboxylate ester thereof
一种异丙烯基、己烯酸的技术,应用在羧酸酯制备、羧酸酯/内酯制备、羧酸酰卤制备等方向,能够解决天然风味影响等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0171] The present invention will be further described with reference to the following Synthesis Examples, Examples and Comparative Examples. It should be understood that the present invention is not limited or limited by these examples.
[0172] Unless otherwise specified, the term "purity" as used herein refers to area percent in gas chromatography (hereinafter also referred to as "GC"). The term "production ratio" refers to the ratio of area percentages in a GC.
[0173] The term "yield" is calculated based on area percent determined by GC.
[0174] Taking into account the purity (%GC) of the starting material and product, the yield was calculated according to the following equation.
[0175] Yield (%)={[(weight of product obtained by reaction×%GC) / molecular weight of product]÷[(weight of starting material in reaction×%GC) / molecular weight of starting material]}×100
[0176] The term "conversion" is calculated from the sum of the area percentages of the starting material...
Synthetic example 1
[0184] Synthesis Example 1: Preparation of 2-methyl-3-buten-2-yl 3-methyl-2-butenoate (3)
[0185]
[0186] The air in the reactor equipped with a stirrer, condenser and thermometer was purged with nitrogen. Then, 3-methyl-2-butenoic acid (100.1 g: 1.00 mol), p-toluenesulfonyl chloride (247.9 g: 1.30 mol) and toluene (300.0 g) were added to the reactor, and heated to 50°C. Pyridine (300.6 g: 3.8 mol) was added dropwise to the reaction mixture for 2 hours at a reaction mixture temperature of 50°C to 60°C. After the dropwise addition was complete, the reaction mixture was stirred at a reaction mixture temperature of 60°C for 1 hour. Then, 2-methyl-3-buten-2-ol (103.4 g: 1.2 mol) was added dropwise to the reaction mixture over 1 hour at a reaction mixture temperature of 65°C to 70°C. The reaction mixture was then stirred at a reaction mixture temperature of 68°C to 70°C for 8 hours. Then, the temperature of the reaction mixture was lowered to 55 to 60°C. Water (185.0 g) wa...
Synthetic example 2
[0192] Synthesis Example 2: Preparation of 2-methyl-3-buten-2-yl 3-methyl-2-butenoate (3)
[0193]
[0194] The procedure of Synthesis Example 1 was repeated except that benzenesulfonyl chloride (229.6 g: 1.30 mol) was used instead of p-toluenesulfonyl chloride to obtain 2-methyl-3-buten-2-yl3-methyl-2- Butenoate (3) (149.7 g: 0.89 mol, yield 89.1%, purity 95.5%).
[0195] Various spectral data of the thus prepared 2-methyl-3-buten-2-yl 3-methyl-2-butenoate (3) were the same as those obtained in Synthesis Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com