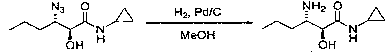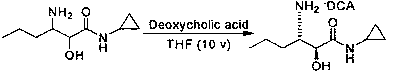Method for preparing 3-amino-N-cyclopropyl-2-hydroxyhexanamide
A technology of hydroxycaproamide and cyclopropyl is applied in the field of preparation of pharmaceutical intermediates, which can solve the problems of difficult industrialized operation, harsh reaction conditions, and high raw material prices, and achieves the advantages of cheap and easy-to-obtain raw materials, mild reaction conditions, and simple operation technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
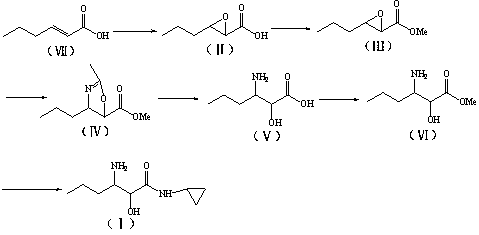
[0043] Example 1Add 400g of water in a 1L reaction flask, add trans-2-hexenoic acid (45.7g, 0.4 mol) and cetylpyridinium chloride (13.6g, 0.04 mol) under stirring, and adjust the pH with 30% aqueous sodium hydroxide solution To 6, add 30% hydrogen peroxide (91 g, 0.8 mol) at room temperature, then heat to 50 ° C for 6 hours until the reaction of the raw materials is complete, cool to room temperature, adjust the pH to 2 with concentrated hydrochloric acid, extract twice with 400 g of dichloromethane, and concentrate To dryness, 48.6g was obtained with a yield of 92%.
[0044] In the above implementation, the lye is replaced by ammonia water, sodium carbonate solution, potassium carbonate solution, potassium hydroxide solution, sodium bicarbonate solution or potassium bicarbonate solution, and the catalyst is sodium tungstate or 12-phosphotungstic acid instead of west Pyridium chloride all can obtain above-mentioned same technical effect.
example 2
[0045] Example 2 Add 130g of water into a 1L reaction flask, add trans-2-hexenoic acid (45.7g, 0.4 mol) and sodium tungstate (26.4g, 0.08mol) while stirring, adjust the pH to 5 with aqueous sodium carbonate solution, and 30% hydrogen peroxide (91 g, 0.8 mol) was added. Then heated to 60°C for 5 hours until the reaction of the raw materials was complete, cooled to room temperature, adjusted the pH to 1 with concentrated hydrochloric acid, extracted twice with 400 g of dichloromethane, and concentrated to dryness to obtain 47.5 g with a yield of 90%.
example 3
[0046] Example 3 Add 400g of water in a 1L reaction flask, add trans-2-hexenoic acid (45.7g, 0.4 mol) and cetylpyridinium chloride (20.4g, 0.06 mol) under stirring, and adjust the pH with 30% aqueous sodium hydroxide solution To 6, add 30% hydrogen peroxide (102.3 g, 0.9 mol) at room temperature, then heat to 50 ° C for 5.5 hours until the reaction of raw materials is complete, cool to room temperature, adjust the pH to 2 with concentrated hydrochloric acid, extract twice with 400 g of dichloromethane, Concentrate to dryness to obtain 49.4g, yield 93.5%.
[0047] Preparation of propyl oxirane-2-methyl carboxylate (III)
[0048] Example 4 Add 530g H to a 1L reaction flask 2 O, and started stirring, while adding 3-propyloxirane-2-carboxylic acid (53g, 0.4 mol), adjusted the pH to 8.5 with 30% NaOH aqueous solution, added 265g ethyl acetate, and added Me 2 SO 4 (60.5g, 0.48 mol), the dropwise addition time is about 1 hour, after the addition is complete, continue to rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com