Amhexenoic acid preparation liquid composition
A vigabatrin and liquid technology, which is applied in the direction of drug combination, drug delivery, medical preparations of non-active ingredients, etc., can solve the problems that powders are prone to bacteria, cannot be stored for a long time, and are inconvenient for children to use for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
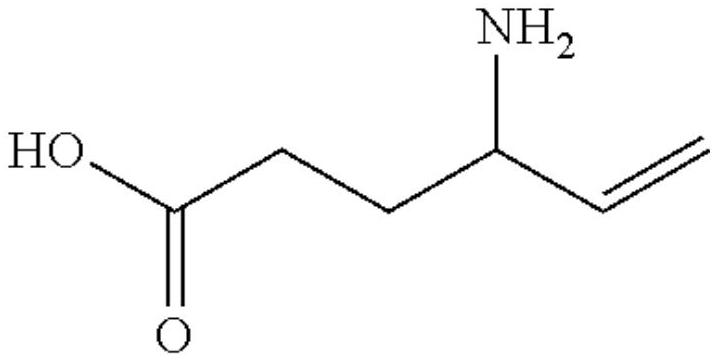
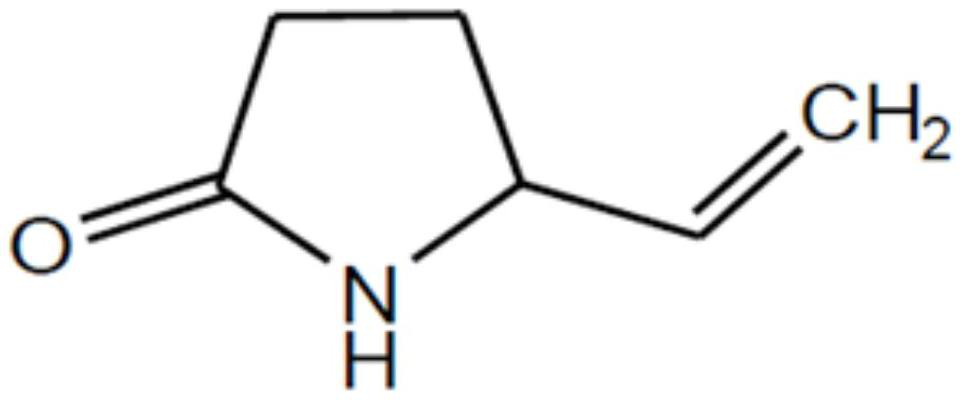
[0059] Example 1: Accurately weigh 500 mg of vigabatrin, 1 g of sucrose, 1 mg of orange essence, 10 mg of methylparaben, 50 mg of Tween 80, and 30 mg of proanthocyanidin, add the above components to 5 mL of water, and then add 0.2M pH Phosphate buffer = 6.8 was made up to 10 mL.
Embodiment 2-8
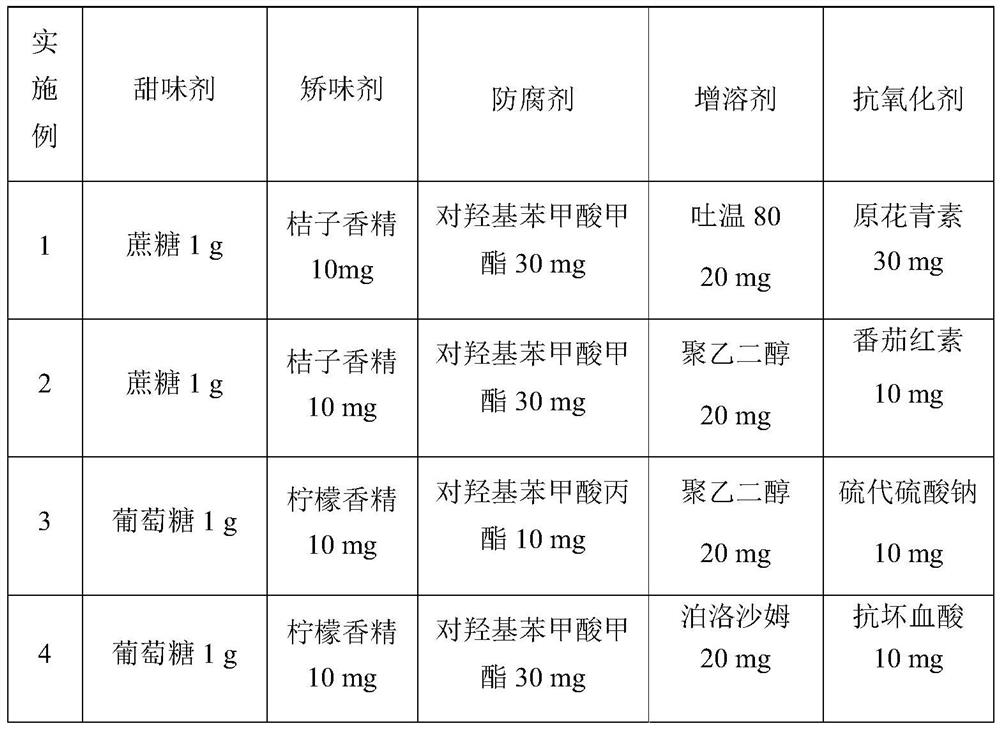
[0060] Other Examples 2-8: In a similar manner to Example 1, other configurations except API and buffer are shown in Table 1 below:
[0061] Table 1 Solution compositions of different groups*
[0062]
[0063]
[0064] (*The above examples all contain 5000 mg of vigabatrin acid, which was made up to 100 mL with 0.2M phosphate buffer at pH=6.8)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com