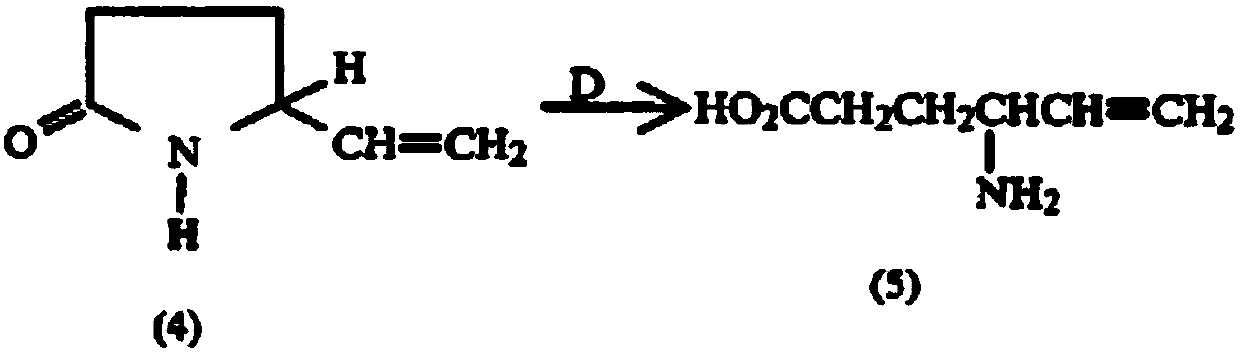
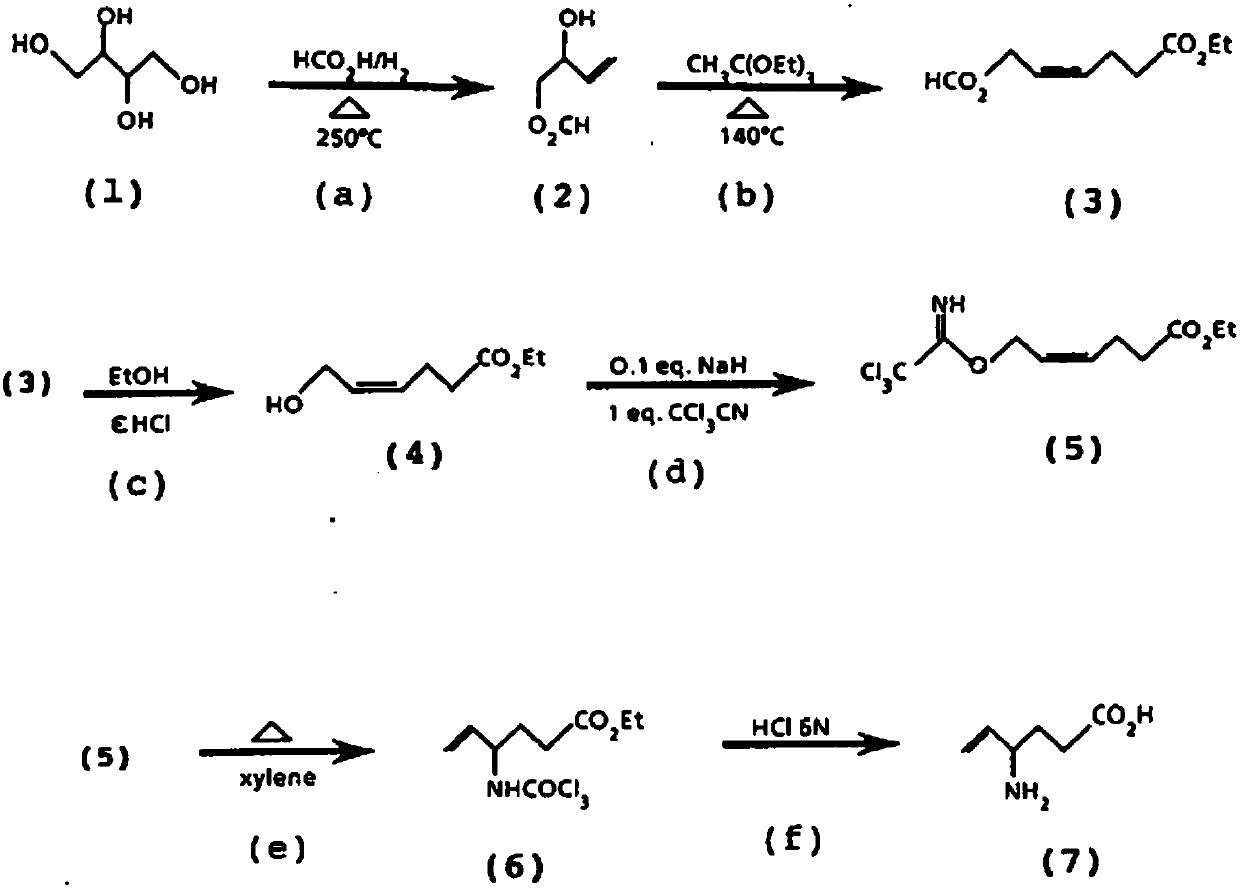
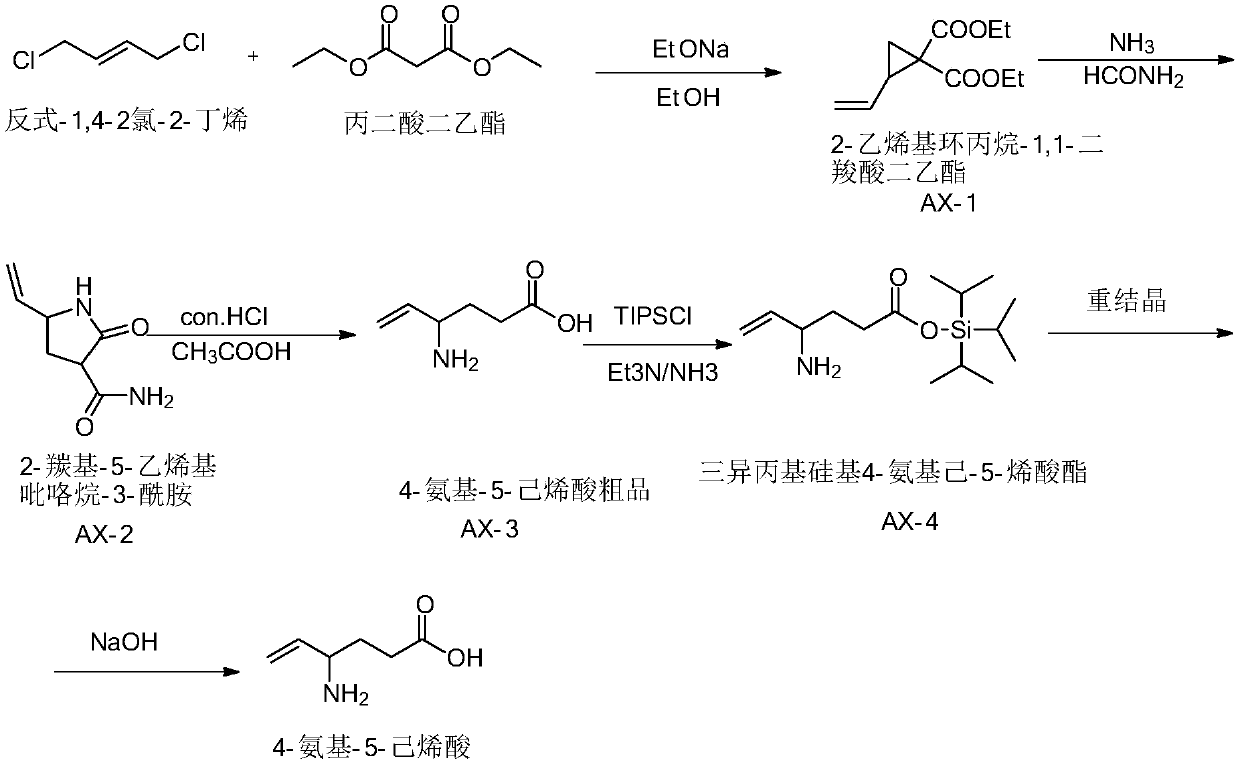
Preparation method of aminohexenoic acid
A technology of aminohexenoic acid and alkenoic acid ester, which is applied in the preparation of carboxylate, the preparation of organic compounds, the preparation of cyanide reaction, etc., can solve the problems such as the inability to obtain high-purity 4-amino-5-hexenoic acid, Achieve the effect of strong industrial practicability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 5L of 20% sodium ethoxide ethanol solution into the enamel reaction kettle, start stirring, and raise the temperature to 55°C; weigh 1kg of diethyl malonate, add it dropwise to the above enamel reaction kettle, add 0.4kg of hydroquinone; Take trans-1,4-dichloro-2-butene, the molar ratio of trans-1,4-dichloro-2-butene to diethyl malonate is 1:1, add it dropwise to the above enamel reaction In the kettle, the dropwise addition was completed in 1.5 to 2 hours; weigh 1L of 20% sodium ethoxide ethanol solution, and add it dropwise to the above-mentioned enamel reaction kettle, and the dropwise addition was completed in 1 to 1.5 hours; the temperature was raised to reflux, and the reaction was performed for 3 hours; the reaction was completed, and the heating was stopped. , cooling down; quenched with water; extracted twice with methyl tert-butyl ether whose volume was 3 times the mass of trans-1,4-dichloro-2-butene, combined the organic layers; Wash twice with sodium chl...
Embodiment 2
[0052] Add 5L of 20% sodium ethoxide ethanol solution into the enamel reaction kettle, start stirring, and raise the temperature to 60°C; weigh 1kg of diethyl malonate, add it dropwise to the above enamel reaction kettle, add 0.5kg of hydroquinone; Take trans-1,4-dichloro-2-butene, the molar ratio of trans-1,4-dichloro-2-butene to diethyl malonate is 1:1, add it dropwise to the above enamel reaction In the kettle, the dropwise addition was completed in 1.5 to 2 hours; weighed 1L of 20% sodium ethoxide ethanol solution, and added dropwise to the above-mentioned enamel reaction kettle, and the dropwise addition was completed in 1 to 1.5 hours; the temperature was raised to reflux, and the reaction was 3.5h; the reaction was completed, stop Heating, cooling down; quenching with water; extracting twice with methyl tert-butyl ether whose volume is 5 times the mass of trans-1,4-dichloro-2-butene, and combining the organic layers; The same amount of base ether was washed with water t...
Embodiment 3
[0059] The operation of preparing AX-4 crude product is the same as that in Example 1.
[0060] Dissolve 500g of triisopropylsilyl 4-aminohex-5-enoate in 8 times volume / mass of chloroform / methanol mixed solution, the volume ratio of chloroform to methanol is 1:7, heat up to 50-60°C, Add cyclohexane dropwise under stirring at a speed of 100rpm, the volume ratio of cyclohexane and chloroform / methanol mixed solution is 1:10, keep stirring for 30min after dropping, drop to 40°C at a speed of 13°C / min, and stir for 30min at a speed of 100rpm , stood still for 2h, and filtered to obtain 485g of purified triisopropylsilyl 4-aminohex-5-enoate, with an HPLC content of 99.01%.
[0061] Dissolve 400g of triisopropylsilyl 4-aminohex-5-enoate purified product in 2L methanol aqueous solution (concentration 50%), add 0.5L sodium hydroxide aqueous solution (concentration 30%), and heat up to 45 ℃, heat preservation reaction for 2 hours, distilled off the water under reduced pressure, and add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com