Process for preparing unsaturated esters
A compound and solvent technology, applied in the field of preparing 4-methyl-6-hex-3-alkenoic acid alkyl ester, can solve problems such as expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
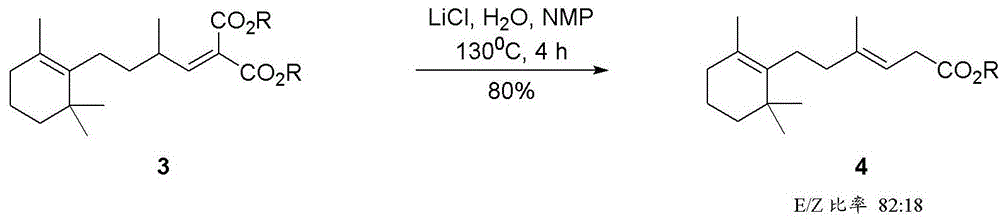
[0035] Preparation of high E ethyl 4-methyl-6-(2,6,6-trimethylcyclohex-1-en-1-yl)hex-3-enoate (4, R=ethyl).
[0036] While stirring, 2-methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)butanal (1) (10 g, 79% purity), dimethylethyl Amide (12 g), monoethyl malonate (8 g, Alfa Aesar 96% purity) and MgCl 2 (2.75g Sigma, anhydrous, >98%) was heated to 120°C and then stirred at this temperature for 2 hours. The yield in terms of purity according to GC analysis was 81% for the E-4 isomer and 11% for the Z-4 isomer (ratio E / Z=88:12). The sum of the isomers was 75% based on the internal standard.
[0037] Cool the reaction mixture to 60 °C and separate the lower MgCl 2 - DMAC phase, the organic phase is washed with water and distilled at 120-130° C. / 0.1 mm.
[0038] After distillation, the E isomer content was 71% and the Z isomer content was 9.5%.
Embodiment 2
[0040] Preparation of high E ethyl 4-methyl-6-(2,6,6-trimethylcyclohex-1-en-1-yl)hex-3-enoate (4, R=ethyl).
[0041] 2-Methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)butanal (1) (10g, 79% purity), dimethylacetamide (10g) , monoethyl malonate (5.5 g) and LiCl (2 g) were heated to 130° C. for 1 hour. Monoethyl malonate (9 g) was then added at 130°C for 3 hours. The reaction mixture was cooled and stirred with water (50 g) and hexane (15 ml). The organic phase was separated and washed 3 times with 20 ml of water, then the solvent was evaporated under reduced pressure to provide a compound containing 52% E-4 isomer, 9.5% Z-4 isomer and 10% conjugated isomer 6 according to GC analysis. 12g of crude product. The yield of the E isomer was 59% in purity.
Embodiment 3
[0043] Preparation of 4-methyl-6-(2,6,6-trimethylcyclohex-1-en-1-yl)hex-3-enoic acid (4, R=H) with high E
[0044] 2-Methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)butanal (1) (10g, 79% purity), dimethylacetamide (12g) , malonic acid (8g) and MgCl 2 (3 g) was heated to 130°C and held for 2 hours.
[0045] According to GC analysis, the reaction mixture contained 39.6% of E-monocyclic homofarnesic acid (4, R=H) and 14.5% of the corresponding Z-4 isomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com