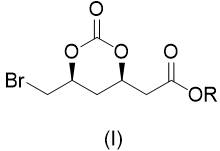
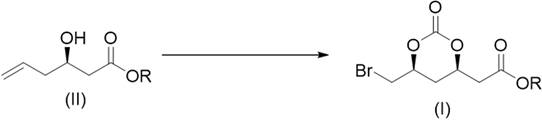
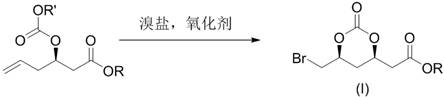
Preparation method of 2-((4r,6s)6 bromomethyl 2-oxo-1,3-dioxane-4-yl)acetate
A technology of dioxane and bromomethyl, applied in the field of chemical synthesis, can solve the problems of high cost, complicated synthesis of substituted oxyformyl protecting groups, poor atom economy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] will( R ) Tert-butyl-3-hydroxy-5-hexenoate (1.86 g), potassium bromide (2.38 g) and dichloromethane (20 mL) are placed in an autoclave and filled with CO 2 (0.5 MPa) gas, add tert-butyl hypochlorite (1.63 g) dropwise under stirring at -20℃, after dripping, keep stirring for 60 minutes, after the reaction is complete, add 10% sodium sulfite solution (15 mL), and use two Extract with methyl chloride, combine the organic phases, wash with saturated sodium bicarbonate solution, dry with anhydrous sodium sulfate, and concentrate to obtain a light yellow solid crude product. The crude product was washed with petroleum ether (25 mL) to obtain a white powdery solid 2-((4 R , 6 S ) Tert-Butyl-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate (2.62 g, 85%).
Embodiment 2
[0028] ( R )-3-Hydroxy-5-hexenoic acid methyl ester (1.44 g), potassium bromide (2.38 g) and acetonitrile (20 mL) were placed in an autoclave and filled with CO 2 (2MPa) gas, add tert-butyl hypochlorite (2.17 g) dropwise with stirring at 0℃, after dripping, keep stirring for 30 minutes, after the reaction is completed, add 10% sodium sulfite solution (15 mL), and use ethyl acetate for the reaction solution Extract, combine the organic phases, wash with saturated sodium bicarbonate solution, dry with anhydrous sodium sulfate, concentrate, and wash the remaining oil with petroleum ether (25 mL) to obtain a pale yellow oil 2-((4 R , 6 S ) Methyl-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate (2.09 g, 75%).
Embodiment 3
[0030] will( R ) Tert-Butyl-3-hydroxy-5-hexenoate (1.86 g) was dissolved in dichloromethane (20 mL), sodium bromide (2.06 g) was added, and CO was introduced 2 (0.1 MPa) gas, add isopropyl hypochlorite (1.90 g) dropwise with stirring at 25°C. After dripping, keep stirring for 45 minutes, after the reaction is completed, add 10% sodium sulfite solution (15 mL), and use dichloride for the reaction solution Methane extraction, combined organic phases, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and concentrated to obtain a light yellow solid crude product. The crude product was washed with petroleum ether (25 mL) to obtain a white powdery solid 2-((4 R , 6 S ) Tert-Butyl-6-bromomethyl-2-oxo-1,3-dioxane-4-yl)acetate (2.47 g, 80%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com