Antigenic peptides for prevention and treatment of cancer
A technology of antigenic peptides and peptides, applied in various peptide fields of immunotherapy and cancer immunotherapy, can solve the problems of limited number of human tumor antigens and autoimmune side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0605] Example 1: Antigenic peptides have superior binding affinity compared to human peptides.
[0606] Computer simulations (in silico) predicted the binding affinities of exemplary antigenic peptides of the invention and corresponding human tumor antigen (human reference peptide) fragments to MHC class I.
[0607] This prediction has been made by using the NetMHC 4.0 server (http: / / www.cbs.dtu.dk / services / NetMHC / ) and as Andreatta M, Nielsen MGapped sequence alignment using artificialneural networks: application to the MHC class I system. Bioinformatics( 2016) Feb 15;32(4):511-7. This approach generates high accuracy predictions of major histocompatibility complex (MHC): peptide binding, specifically peptides 8-11 amino acids in length.
[0608] Table 2 below shows the results, ie information on the prediction of peptide-MHC class I binding.
[0609]
[0610]
[0611]
[0612]
[0613]
[0614]
[0615]
[0616]
[0617]
[0618]
[0619] ...
Embodiment 2
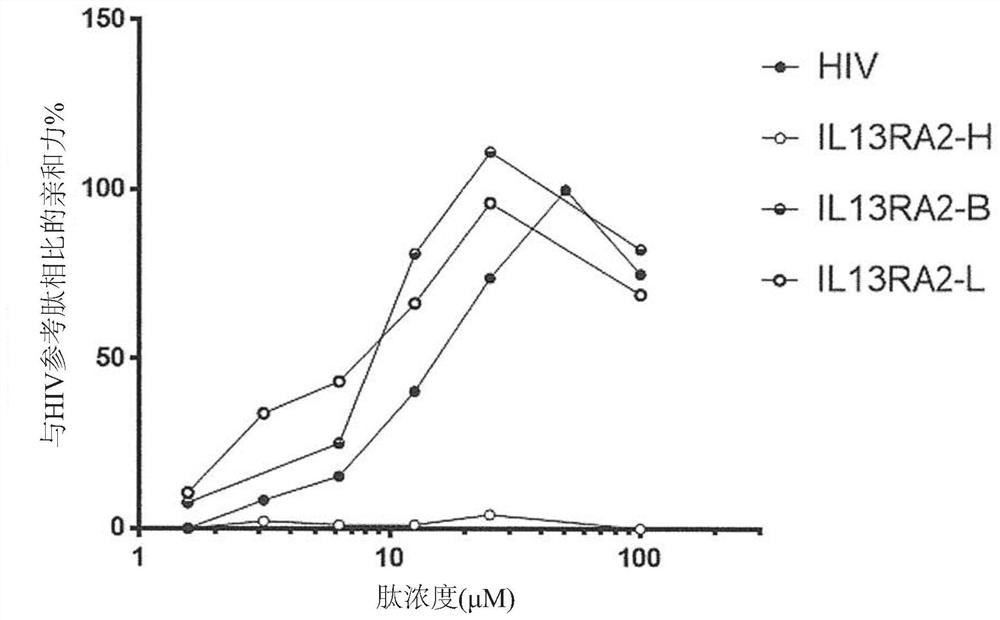
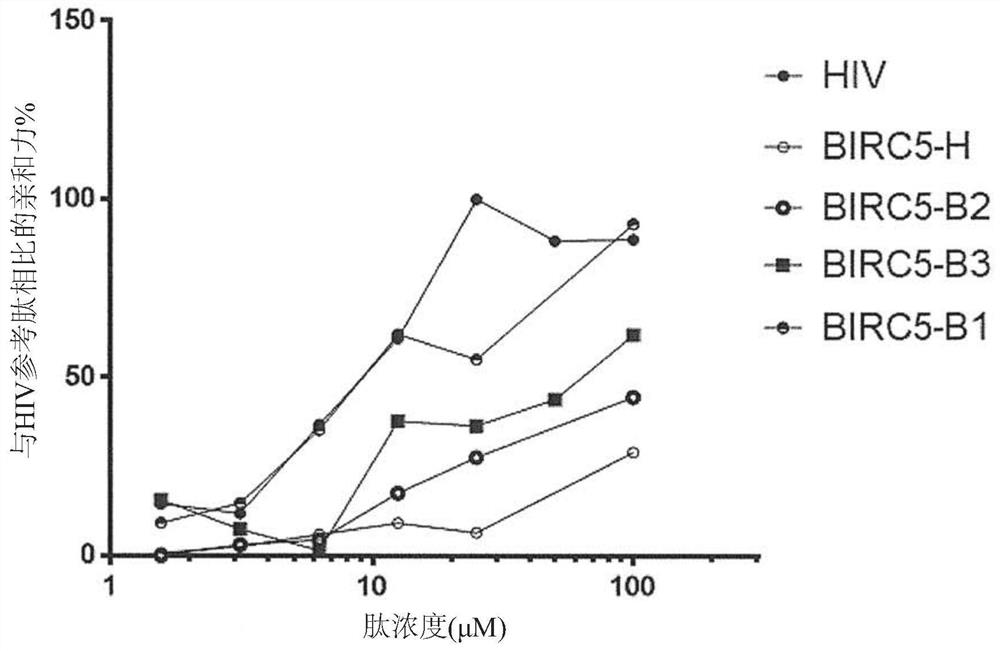
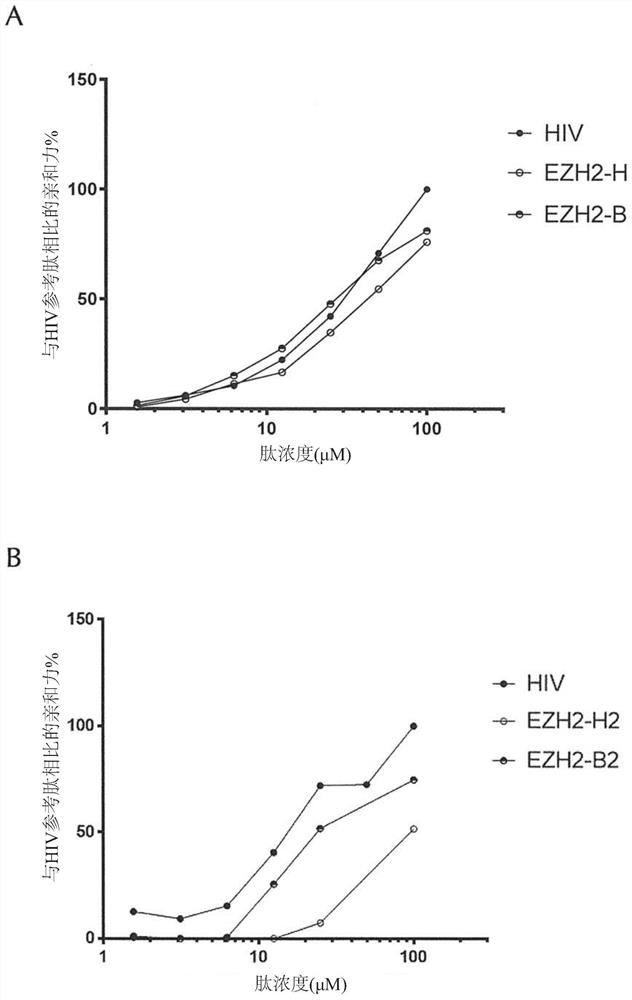
[0627] Example 2: Antigenic peptides have superior affinity to HLA-A*0201 alleles.
[0628] Then, the binding affinity of each selected antigen peptide and the corresponding human tumor antigen (human reference peptide) fragment to the HLA-A*0201 allele was confirmed in vitro. That is, the sequence SEQ ID NO:32 ("FMLGEFLKL", also referred to herein as BIRC5-B1); SEQ ID NO:30 ("YTLGEFLYI", also referred to herein as BIRC5-B2); and SEQ ID NO :31 ("GLLGEFLQI", also referred to herein as BIRC5-B3) antigenic peptide was compared with the corresponding reference human peptide derived from BIRC5 ("LTLGEFLKL", SEQ ID NO: 593, also referred to herein as BIRC5-H) Compare. In addition, the antigenic peptide of the sequence SEQ ID NO:97 ("LLLSAALSV", also referred to herein as CHI3L1 B); and SEQ ID NO:87 ("YLLSAALTI", also referred to herein as CHI3L1 B3) is identical to that derived from CHI3L1 ("LLLSAALSA", SEQ ID NO: 617, also referred to herein as CHI3L1 H) for comparison. In add...
Embodiment 3
[0648] Example 3: In the ELISPOT-IFNγ assay, vaccination of mice with antigenic peptides according to the invention induces Enhanced T cell response.
[0649] a. Materials and methods
[0650] A.1 Mouse model
[0651] The immunization protocol is shown in Figure 7 middle. Briefly, HLA-A2 humanized mice (HLA-A2(CB6F1-Tg(HLA-A*0201 / H2-K b)A*0201) were randomly assigned (based on mouse sex and age) to experimental groups, where each group was treated with the same helper peptide (h-pAg T13L; sequence: TPPAYRPPNAPIL; SEQ ID NO: 860; Bhasin M, Singh H, Raghava GP (2003) MHCBN: a comprehensive database of MHC binding and non-binding peptides. Bioinformatics 19:665–666) (summarized in Table 5 below) combined specific vaccination peptide (vacc-pAg) immunization. The vacc-pAg was compared in pairs (Group 1 vs. Group 2, Group 1 vs. Group 3; Group 1 vs. Group 4; Group 5 vs. Group 6; Group 7 vs. Group 8; Group 9 vs. Group 10). Thus, each wave compares native and optimized for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com