Antibodies targeting glycoprotein vi
An antibody and antibody fragment technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., can solve problems such as lack of cross-reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0107] sequence
[0108] In one embodiment, the present disclosure relates to antibodies or antibody fragments specific for glycoprotein VI (GPVI). In one embodiment, the present disclosure relates to isolated antibodies or antibody fragments specific for glycoprotein VI (GPVI). In one embodiment, the present disclosure relates to isolated monoclonal antibodies or antibody fragments specific for GPVI. In one embodiment, the present disclosure relates to an isolated monoclonal antibody or antibody fragment specific for GPVI comprising the variable heavy chain (VH) and variable light chain (VH) of any one of the antibodies disclosed in Tables 1-4 VL), in one embodiment, the present disclosure relates to an isolated monoclonal antibody or antibody fragment specific for GPVI comprising the heavy chain (HC) and light chain (LC) of any one of the antibodies disclosed in Tables 1-4 ).
[0109] In one embodiment, the present disclosure relates to an isolated monoclonal antibody or ...
Embodiment 13
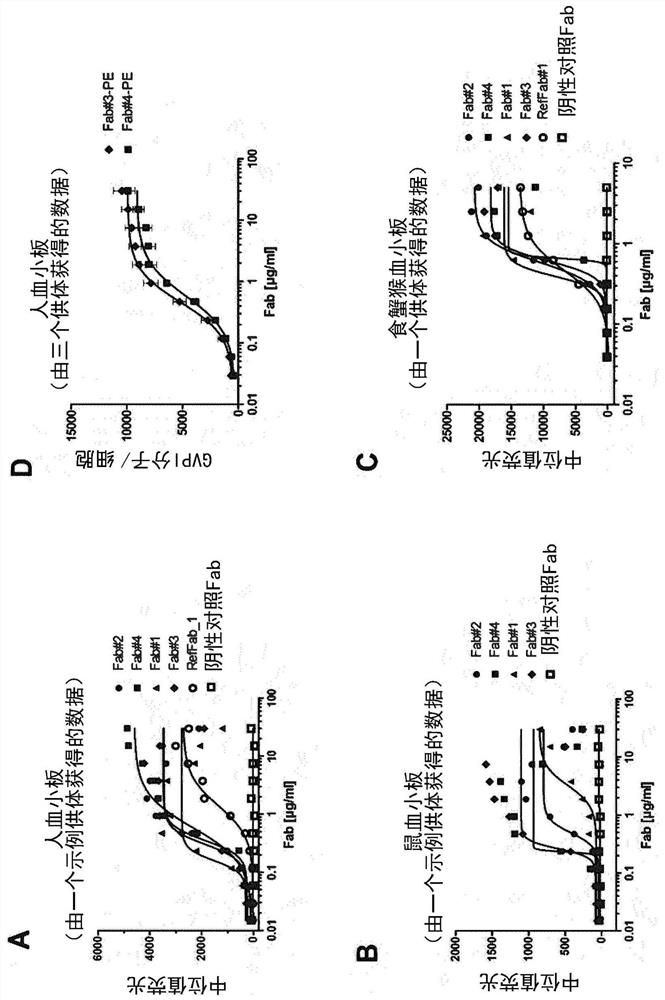
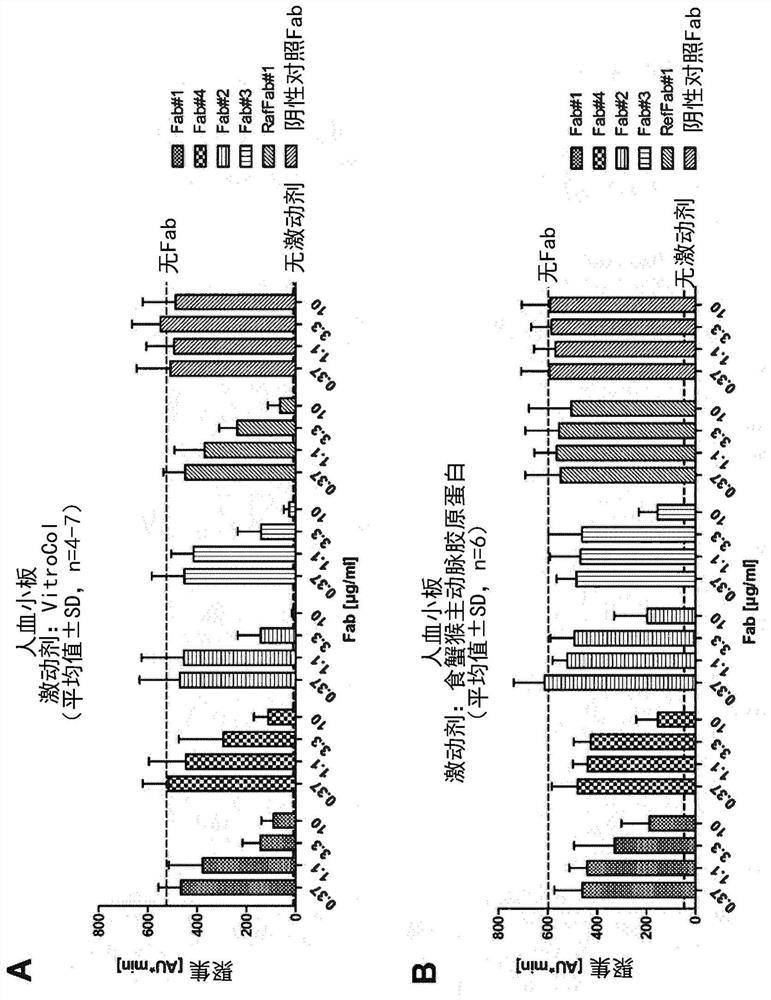
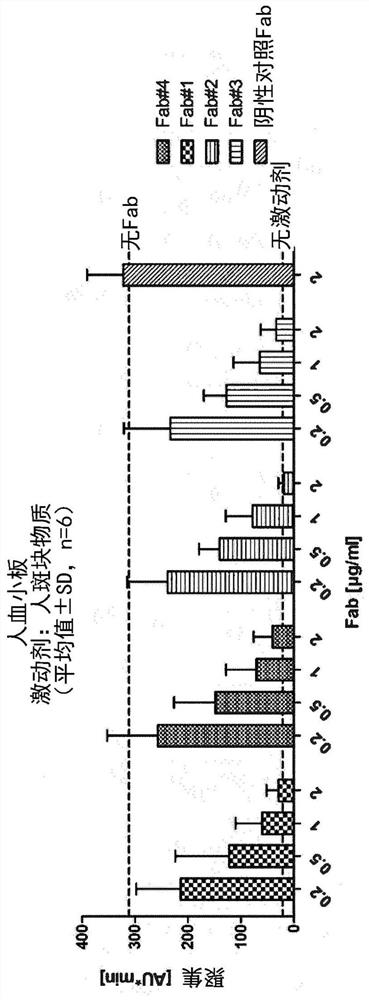
[0493] Thus, in one embodiment, a Fab specific for GPVI of the present disclosure does not substantially activate platelets due to recognition by anti-Fab antibodies present in the subject's serum. In another embodiment, the Fabs of the disclosure specific for GPVI do not substantially induce the expression of cell surface activation markers such as CD62P on platelets.
[0494] The inventors of the present application have confirmed that modification of the C-terminus of the Fab heavy chain can further reduce, prevent or inhibit the possibility of this Fab-induced platelet activation, for example by preventing the anti-Fab antibodies present in the subject's serum from recognizing the GPVI-specific Fab.
[0495] Herein, "prevent recognition" or "prevent binding" means that the Fabs specific for GPVI of the present disclosure do not cross-link GPVI on the platelet surface and activate GPVI signaling due to the presence of anti-Fab antibodies in the subject's serum. This cross-...
Embodiment 1
[0667] Example 1: Antigen Production and Quality Control
[0668] The amino acid sequences of GPVI for human, cynomolgus monkey, mouse and rat were retrieved from publicly available sources (eg Uniprot) and produced in-house.
[0669] recombinant soluble extracellular domain
[0670] Soluble GPVI corresponds to the extracellular domain of GPVI fused at its C-terminus to a human Fc sequence. This soluble GPVI can be referred to as GPVI-Fc.
[0671] Human GPVI-1A (SEQ ID NO: 4), human GPVI-1B (SEQ ID NO: 5), human GPVI-2A (SEQ ID NO: 6), cynomolgus monkey GPVI (SEQ ID NO: 7), mouse The extracellular domain (ECD) of GPVI (SEQ ID NO: 8) and rat GPVI (SEQ ID NO: 9) was cloned into the expression vector pMAX_vk_Fc2 (K105-K330) using KpnI and EcoRV to generate the C-terminal Fc2 (K105- K330) fusion construct. Fc2(K105-K330) has the amino acid sequence disclosed in SEQ ID NO:10.
[0672] The DNA encoding the extracellular domain of human, cynomolgus monkey, mouse or rat GPVI wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com