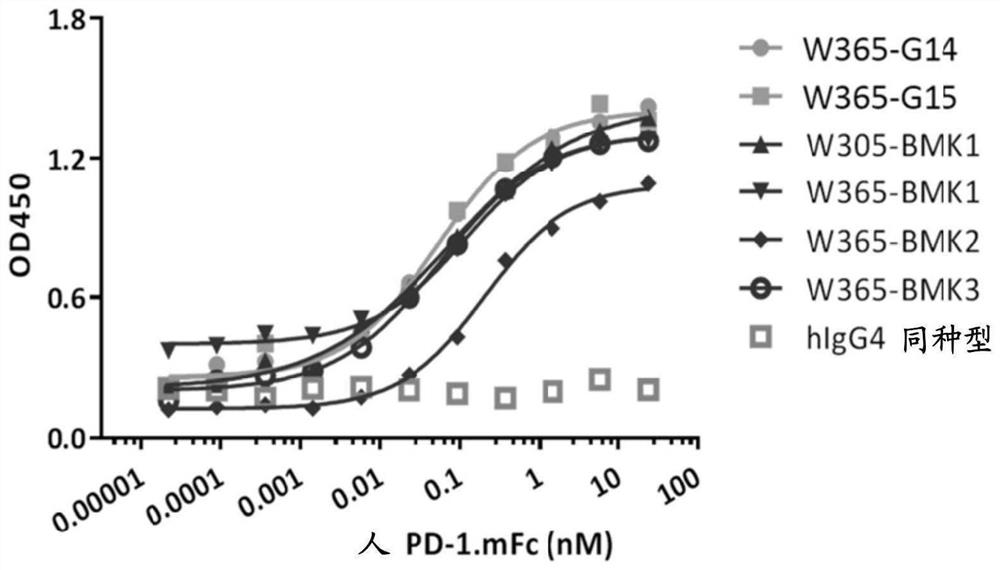
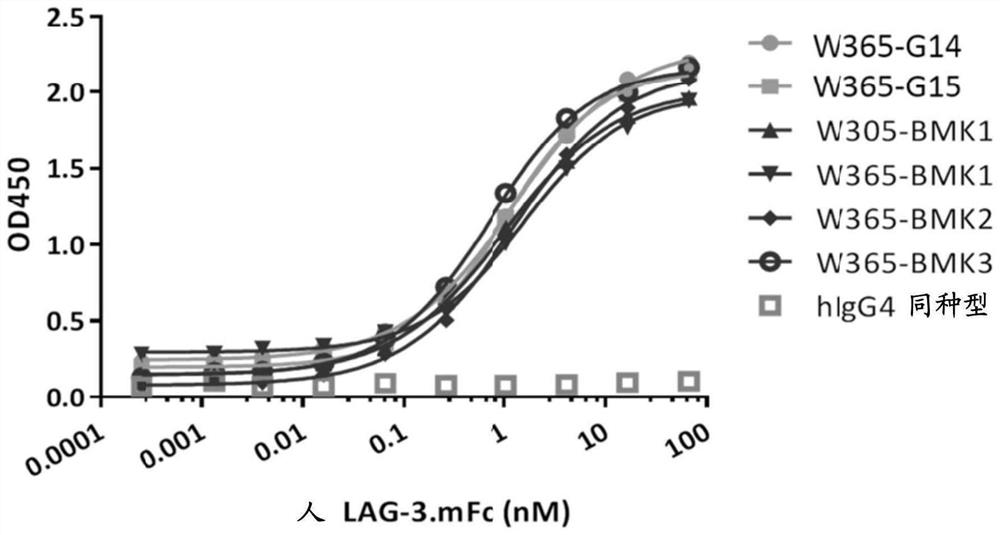
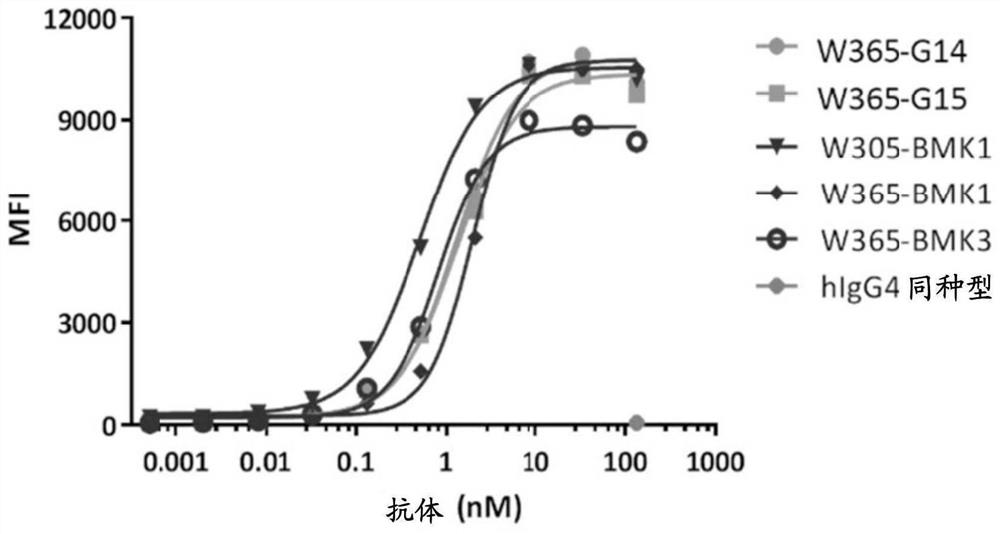
Novel bispecific pd-1/lag-3 antibody molecules
A bispecific antibody, LAG-3 technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc., can solve problems such as defects in down-regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0324] Example 1: Generation and Characterization of W3055-1.153.7 Monoclonal Antibody
[0325] Fully human W3055-1.153.7 with a heavy chain variable region of SEQ ID NO: 17, a kappa light chain variable region of SEQ ID NO: 18, and human IgG4 was obtained as described in PCT Application No.: PCT / CN2016 / 094624 constant region. As disclosed in PCT Application No. PCT / CN2016 / 094624, the affinity of W3055-1.153.7 for recombinant human PD-1 by SPR is 2.79 nM. W3055-1.153.7 bound cynomolgus monkey PD-1 but not murine PD-1 as determined by FACS. W3055-1.153.7 specifically binds PD-1, but not CD28 and CTLA4 of the PD-1 family. The results of SPR assay and FACS of binning test showed that the epitope on human PD-1 bound by W3055-1.153.7 was different from that of existing PD-1 antibody (i.e. the benchmark antibody nivolumab (5C4 clone of BMS patent US9084776B2) and pembrolizumab (disclosed as clone hPD-1.09A in US8354509B2 and WO2008156712A1).[ 3H] Thymidine incorporation assay sh...
Embodiment 3
[0328] Example 3: Generation and Characterization of Human W3395-3.40.19LAG-3Ab Monoclonal Antibody
[0329] Monoclonal human LAG-3 antibody W3395-3.40.19 was generated as described in PCT / CN2019 / 076356. Typically, OMT rats (transgenic rats with recombinant immunoglobulin loci, as described and produced in US8,907,157B2) are immunized with the human LAG-3 antigen to obtain an immunization in which both the framework and CDR regions are derived from the human germline Antibodies against globulin sequences. Hybridomas generated by fusion of immunized rat lymph nodes and spleens with myeloma cells were isolated, selected and subcloned. Total RNA of hybridomas was extracted, and cDNA was synthesized and amplified. The VH and VL genes were reamplified and cloned into expression vectors to create corresponding clones of the antibodies.
[0330] By FACS, the binding affinity of W3395-3.40.19 to cell surface human LAG-3 had an EC50 value of 0.13 nM, which was much lower than that o...
Embodiment 4
[0335] Example 4. Construction and characterization of bispecific antibodies
[0336] 1. Production of antigens and other proteins
[0337] 1.1 Antigen production
[0338] Nucleic acids encoding human PD-1, human and mouse LAG-3 ECD (extracellular domain) were synthesized by Sangon Biotech. PD-1 or LAG-3 gene fragments were amplified from the synthetic nucleic acids and inserted into the expression vector pcDNA3.3 (ThermoFisher). The inserted PD-1 or LAG-3 gene fragment was further confirmed by DNA sequencing. By transfecting human PD-1 or LAG-3 gene into 293F cells (ThermoFisher), a fusion protein containing human LAG-3 ECD and various tags, including human Fc and mouse Fc, was obtained. Incubate cells at 37 °C, 5% CO 2 Cultured in FreeStyle 293 expression medium. After 5 days of culture, supernatants from cultures of transiently transfected cells were harvested for protein purification. Fusion proteins were purified by protein A and / or SEC columns. Untagged LAG-3 ECD ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com