Lentiviral packaging vector system, lentivirus and its construction method, kit
A technology of lentiviral packaging and vector system, applied in the field of lentiviral packaging vector system, can solve problems such as limiting the efficiency of drug research and development of SARS-CoV-2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
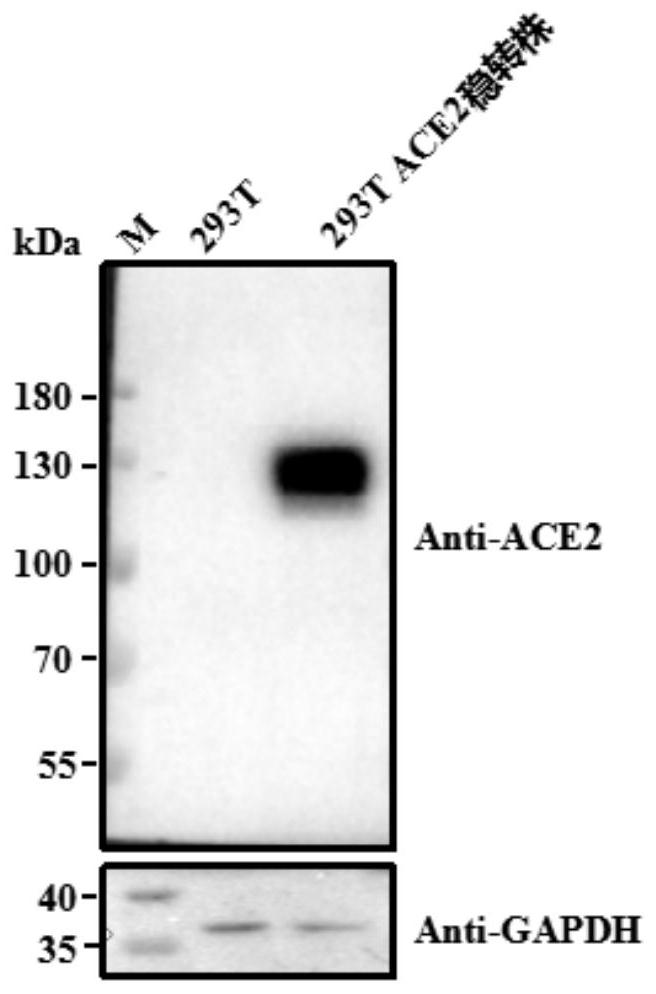
[0042] Preparation of cell lines expressing ACE2 receptors
[0043] (1) pLV[Exp]-Bsd-CMV>hACE2 (from Yunzhou Biology) was packaged into lentivirus with VSV-G;
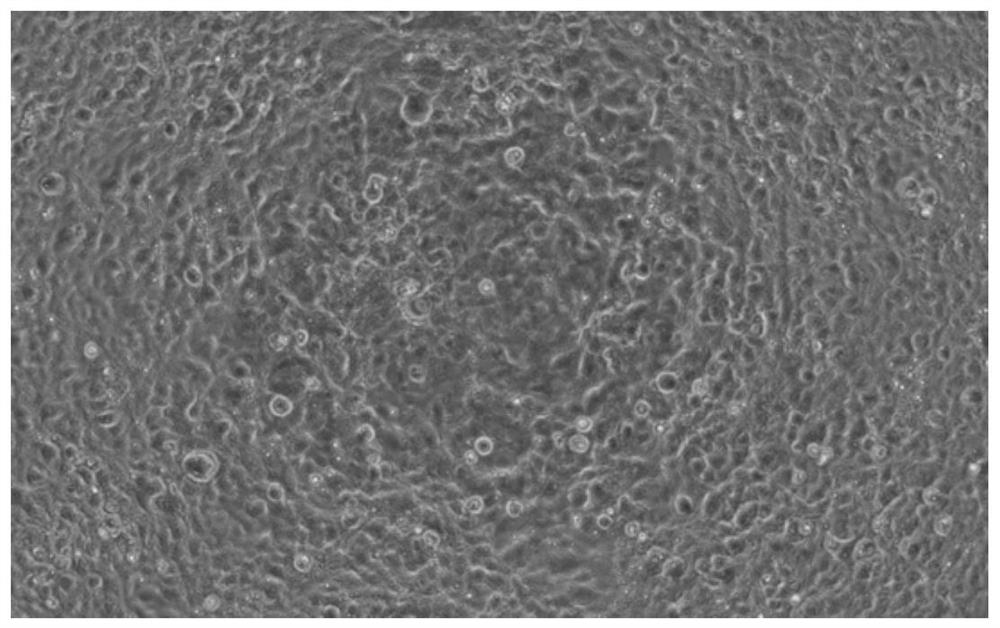
[0044] (2) The lentivirus in step (1) was transduced into HKE293T cells and screened with blasticidin Bsd (Blasticidin) at an appropriate concentration until the blank cells died to obtain a stable transfected strain expressing the ACE2 receptor, denoted as HEK293T- ACE2 stable transfected strain. The image of HEK293T-ACE2 stable transfected strain under 200× microscope is as follows figure 1 shown. Depend on figure 1 It can be seen that the HEK293T-ACE2 stably transformed strain grows well.
[0045] (3) Extract the genomic DNA of HEK293T-ACE2 stable transgenic strain, and perform qRT-PCR detection, wherein the qPCR primers are as follows: ACE2-F: 5'-GCAGCCACACCTAAGCATT-3' (SEQ ID No: 6); ACE2-R: 5' - CCATCCACCTCCACTTCTCT-3' (SEQ ID No: 7); HKE293T as blank control.
[0046] The results of qRT-PCR showed that com...
Embodiment 2
[0050] Detect the expression level of S protein after transient transfection of HEK293T cells with different S protein expression vectors (the vector is used as an envelope protein expression plasmid in the process of virus packaging)
[0051] (1) Transfect HEK293T cells with the same amount of 10 kinds of S protein expression vectors using Lipofectamine 2000 as transfection reagent, respectively, to obtain HEK293T cells corresponding to each S protein expression vector. Among the 10 S protein expression vectors, the nucleotide sequence of S_a is shown in SEQ ID No: 8, encoding the wild-type S protein; the nucleotide sequence of S_c is shown in SEQ ID No: 9, encoding the D614G mutant S Protein; the nucleotide sequence of S_b is shown in SEQ ID No: 10, which is the nucleic acid fragment corresponding to the purification tag 3×Flag after optimization on the basis of S_a (that is, the nucleotide sequence is shown in SEQ ID No: 3). The nucleic acid fragment of S_d+3×Flag correspon...
Embodiment 3
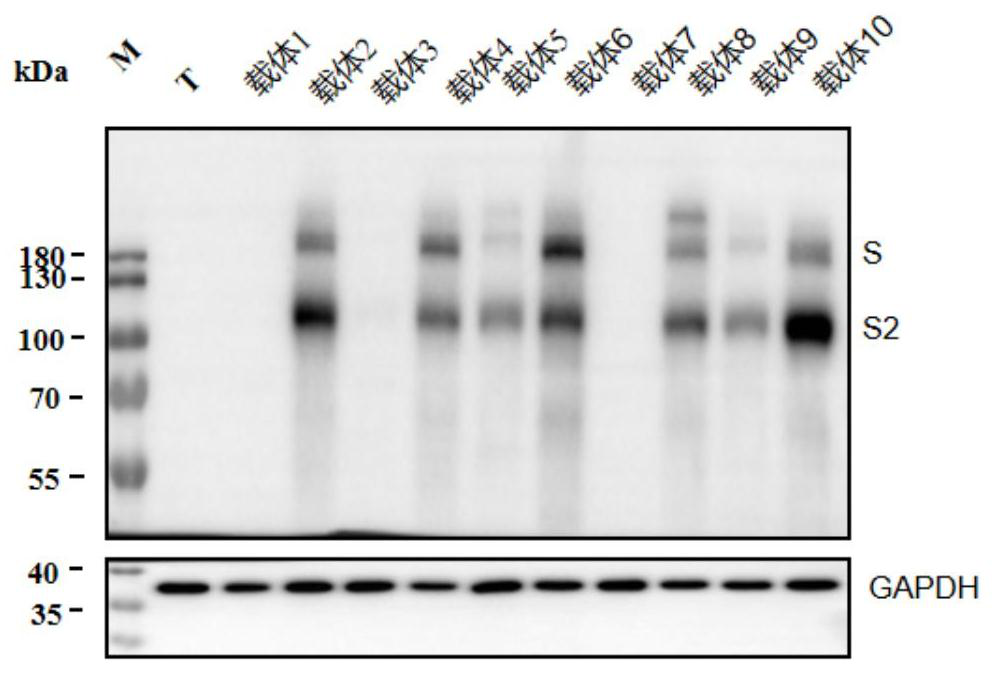
[0058] Comparison of lentiviral packaging effects of different S protein expression vectors
[0059] Part of the S protein expression vector of Example 2 was selected for lentiviral packaging test, and the materials used in the test included the following:
[0060] Vector 1: pRP[Exp]-CMV>S_a; Vector 2: pRP[Exp]-CMV>S_b; Vector 4: pRP[Exp]-CMV-human beta globin intron>S_b; Vector 6: pRP[Exp]-CAG >S_b; Vector 7: pRP[Exp]-CMV-humanbeta globin intron>S_c; Vector 8: pRP[Exp]-CMV-human beta globin intron>S_d; Vector 9: pRP[Exp]-CAG>S_c; Vector 10 : pRP[Exp]-CAG>S_d; lentiviral packaging expression plasmid: pLV[Exp]-CMV>EGFP; gag-pol expression plasmid (hereinafter referred to as PLV4): containing lentivirus gag gene and pol gene; rev expression plasmid ( hereinafter referred to as PLV5): encoding lentivirus Rev regulator; RIPA lysate (strong), Biyuntian P0013B; Sars SpikeProtein Antibody (antibody against SARS-S protein), (novusbio NB100-56578SS); horseradish peroxidase labeled goa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com