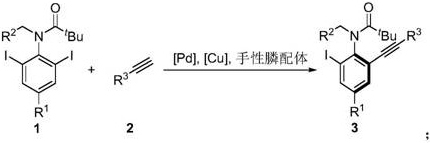
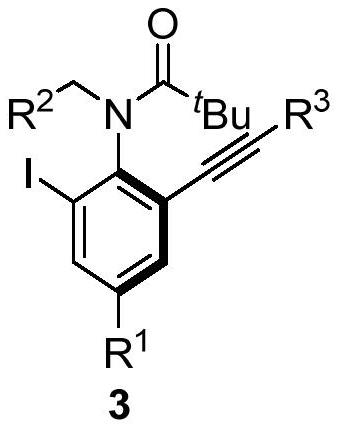
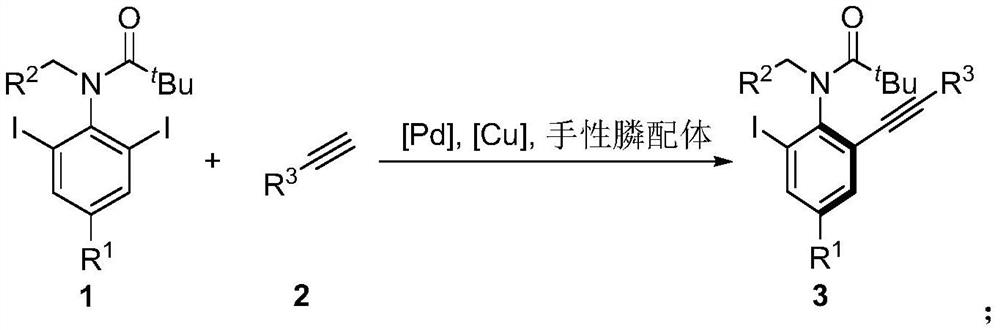
A kind of axial chiral anilide compound and its preparation method and application
An axial chirality and anilide technology, applied in the field of compound synthesis, can solve problems such as difficulty in generalization, and achieve the effect of high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] The specific operation is: under the protection of nitrogen, add Pd(OAc) to the dry Shrek tube 2 (0.02mmol), organophosphine ligand L19 (0.04mmol) and 2ml of anhydrous ethyl acetate, stirred at room temperature for 1h. Then CuI (0.01 mmol), substrate 1a (0.2 mmol), substrate 2a (0.4 mmol) and cesium acetate (0.4 mmol) were added, and stirred at 5° C. for 60 h. The reaction solution was concentrated, and 95.0 mg of product 3aa was obtained by column chromatography, with a yield of 81%, and 97% ee by HPLC.
[0046] Product characterization data are:
[0047] 1H NMR (400MHz, CDCl 3 )δ=7.74(d,J=1.3,1H),7.65–7.59(m,6H),7.48(t,J=7.5,2H),7.44(d,J=1.2,1H),7.39(t,J =7.3,1H),4.05(dd,J=13.6,7.8,1H),2.99(dd,J=13.6,5.2,1H),2.37(s,3H),2.08(d,J=12.2,1H), 1.85–1.73(m,1H),1.68–1.60(m,2H),1.60–1.50(m,2H),1.16(s,9H),1.14–1.05(m,3H),1.04–0.89(m,2H ). 13 C NMR (101MHz, CDCl 3 )δ178.17,145.76,141.56,140.86,140.18,139.11,133.83,132.09,128.91,127.79,127.12,127.05,125.49,121...
Embodiment 2
[0050]
[0051] The specific operation is: under the protection of nitrogen, add Pd(OAc) to the dry Shrek tube 2 (0.02mmol), organophosphine ligand L19 (0.04mmol) and 2ml of anhydrous ethyl acetate, stirred at room temperature for 1h. Then CuI (0.01 mmol), substrate 1a (0.2 mmol), substrate 2b (0.4 mmol) and cesium acetate (0.4 mmol) were added, and stirred at 5° C. for 60 h. The reaction solution was concentrated, and 84.2 mg of product 3ab was obtained by column chromatography, with a yield of 82%, and 96% ee by HPLC.
[0052] Product characterization data are:
[0053] 1 H NMR (400MHz, CDCl 3 )δ7.73(d,J=1.3Hz,1H),7.55–7.50(m,2H),7.42(d,J=1.2Hz,1H),7.38–7.32(m,3H),4.03(dd,J =13.6,7.9Hz,1H),2.96(dd,J=13.6,5.2Hz,1H),2.36(s,3H),2.04(d,J=12.5Hz,1H),1.85–1.69(m,1H) ,1.67–1.57(m,2H),1.55–1.47(m,2H),1.14(s,9H),1.11–1.02(m,3H),1.02–0.85(m,2H). 13 C NMR (101MHz, CDCl 3 )δ178.11,145.76,140.85,139.09,133.85,131.65,128.86,128.45,125.45,122.40,102.69,94.54,87.25,59.87,41.97,37....
Embodiment 3
[0055]
[0056] The specific operation is: under the protection of nitrogen, add Pd(OAc) to the dry Shrek tube 2 (0.02mmol), organophosphine ligand L19 (0.04mmol) and 2ml of anhydrous ethyl acetate, stirred at room temperature for 1h. Then CuI (0.01 mmol), substrate 1a (0.2 mmol), substrate 2c (0.4 mmol) and cesium acetate (0.4 mmol) were added, and stirred at 5° C. for 60 h. The reaction solution was concentrated, and 86.5 mg of product 3ac was obtained by column chromatography, with a yield of 82%, and 96% ee by HPLC.
[0057] Product characterization data are:
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.69(s,1H),7.42–7.33(m,3H),7.14(d,J=7.9Hz,2H),4.01(dd,J=13.6,7.9Hz,1H),2.92(dd,J =13.6,5.1Hz,1H),2.36(s,3H),2.33(s,3H),2.02(d,J=12.4Hz,1H),1.79–1.68(m,1H),1.66–1.56(m, 2H),1.54–1.45(m,2H),1.11(s,9H),1.09–1.00(m,3H),0.98–0.82(m,2H). 13 C NMR (101MHz, CDCl 3 )δ177.99,145.49,140.48,138.99,138.90,133.62,131.43,129.09,125.54,119.21,102.52,94.72,86.56,59.73,41.83,37.35,32.12,29.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com