Application of pedf in preparation of medicine for treating myeloproliferative diseases
A bone marrow proliferation and disease technology, applied in the application field of PEDF in the preparation of drugs for the treatment of myeloproliferative diseases, can solve the problems of inability to reduce the JAK2 gene mutation load, thrombocytopenia, etc., to reduce peripheral blood, inhibit proliferation, and reduce mortality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
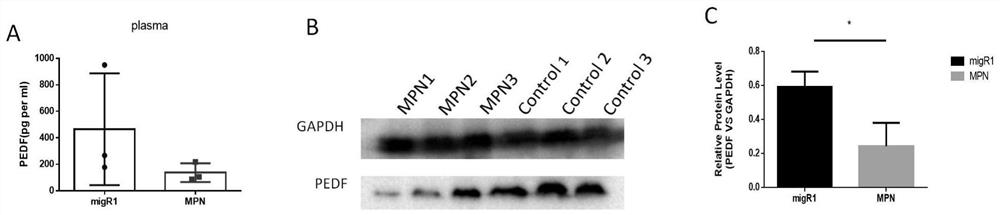
[0032] Reference attached figure 1 The results of enzyme-linked immunosorbent assay and Western blot detection on MPN mice after modeling showed that the expression of PEDF in plasma and bone marrow cells of MPN mice decreased. A: Concentration of PEDF in plasma of control group and MPN mice; B, C: Expression of PEDF protein in bone marrow mononuclear cells of control group and MPN mice. (n=3, *P<0.05)
Embodiment 2
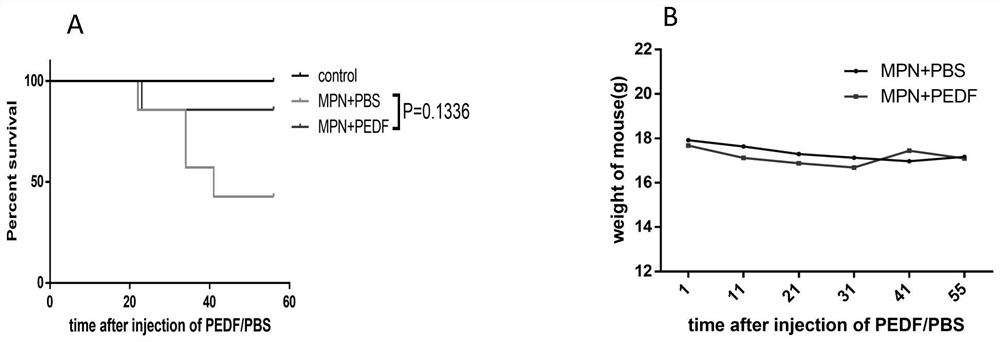
[0034] Reference attached figure 2, MPN mice (n=7) were intervened with PBS or PEDF for 8 weeks, 4 mice given PBS died, and 1 mouse given PEDF died. According to the results of the survival rate, compared with the MPN mice in the control solvent PBS group, the death rate of the MPN mice in the PEDF group decreased, indicating that the PEDF treatment prolongs the survival time of the MPN mice.
Embodiment 3
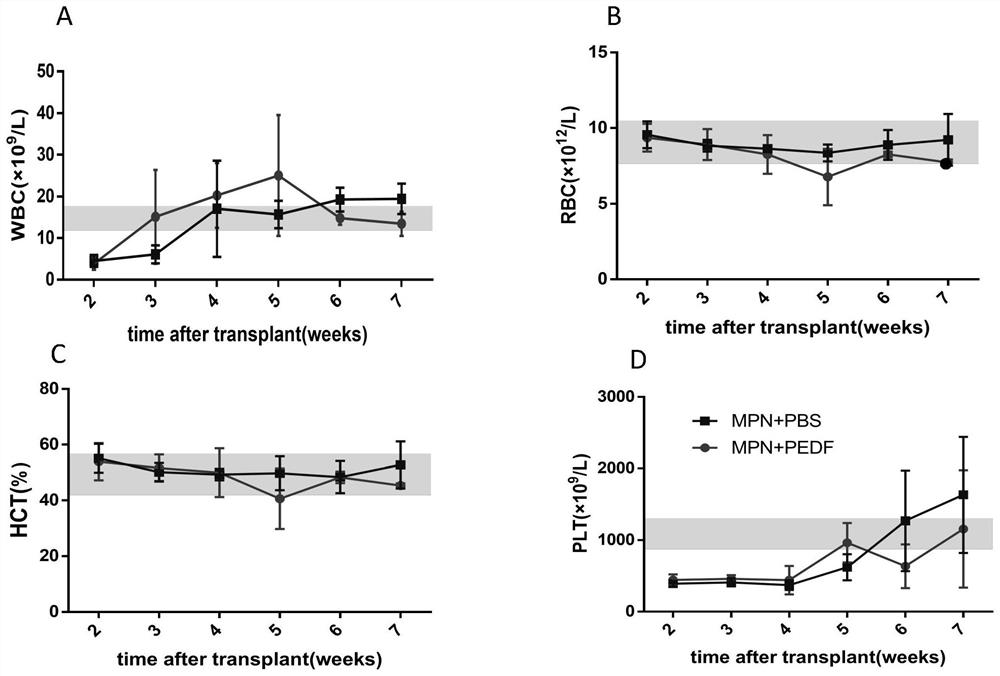
[0036] Reference attached image 3 , MPN mice were treated with PBS / PEDF for five weeks, and blood was collected from the tail vein every week. The results of hemocytometer showed that compared with the MPN mice treated with PBS, the WBC, RBC, HCT, PLT decreased, indicating that the infusion of PEDF reduced the peripheral blood of MPN mice. The average values of peripheral blood of mice in each group are shown in Table 1.
[0037] Table 1
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com