Application of cannabidiol in preparation of medicine for treating acute B lymphocytic leukemia
A technology of B lymphocytes and cannabidiol, which is applied in the field of biomedicine and can solve the problems that cannot be used to predict efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
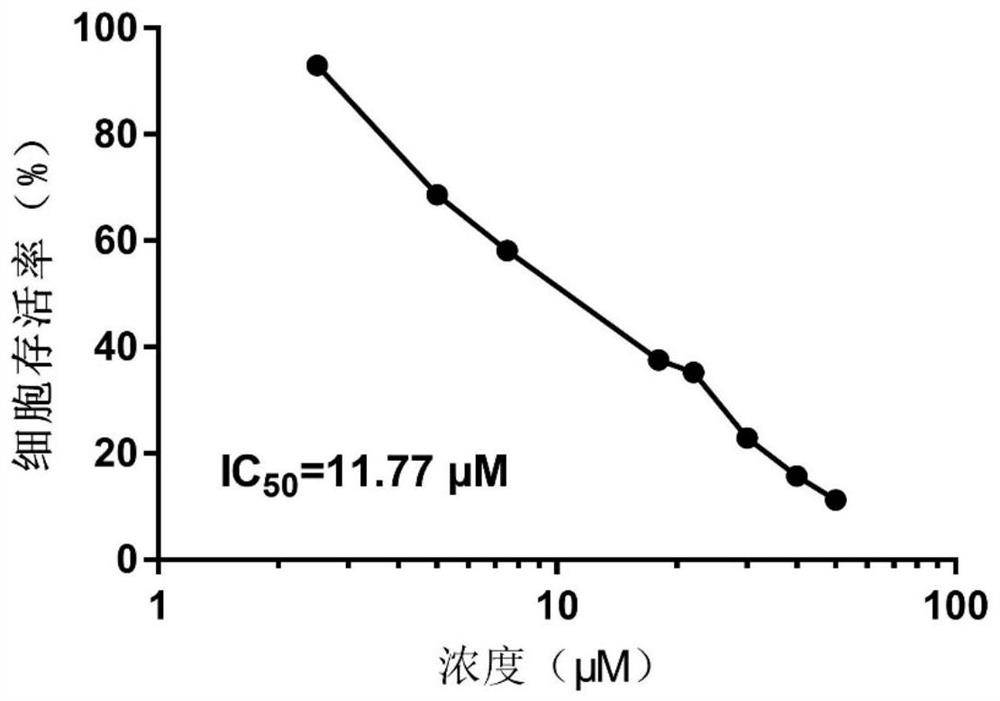
[0033]Embodiment 1, the inhibitory effect of cannabidiol (CBD) on the survival of human acute lymphoblastic leukemia cell Nalm-6 in vitro
[0034] Cannabidiol (CBD) was dissolved in DMSO to a concentration of 50 μM and stored at -20°C until use. Cannabidiol (CBD) was further diluted to the required concentration before use, and the DMSO concentration was determined to be less than 0.001%. The human acute B-lymphoblastic leukemia cell Nalm-6 used in this experiment was used as the test cell. The cells were cultured at 37°C in a humid atmosphere with 5% CO2 and 95% air, and the culture medium was RPMI-1640 containing 10% heat-inactivated fetal bovine serum. The survival rate of human acute B-lymphoblastic leukemia cells Nalm-6 at different concentrations of cannabidiol (CBD) was tested.
[0035] result: figure 1 Describes the survival of Nalm-6 human acute lymphoblastic leukemia cells when cannabidiol (CBD) is administered. It can be seen that as the dose of cannabidiol (CBD...
Embodiment 2
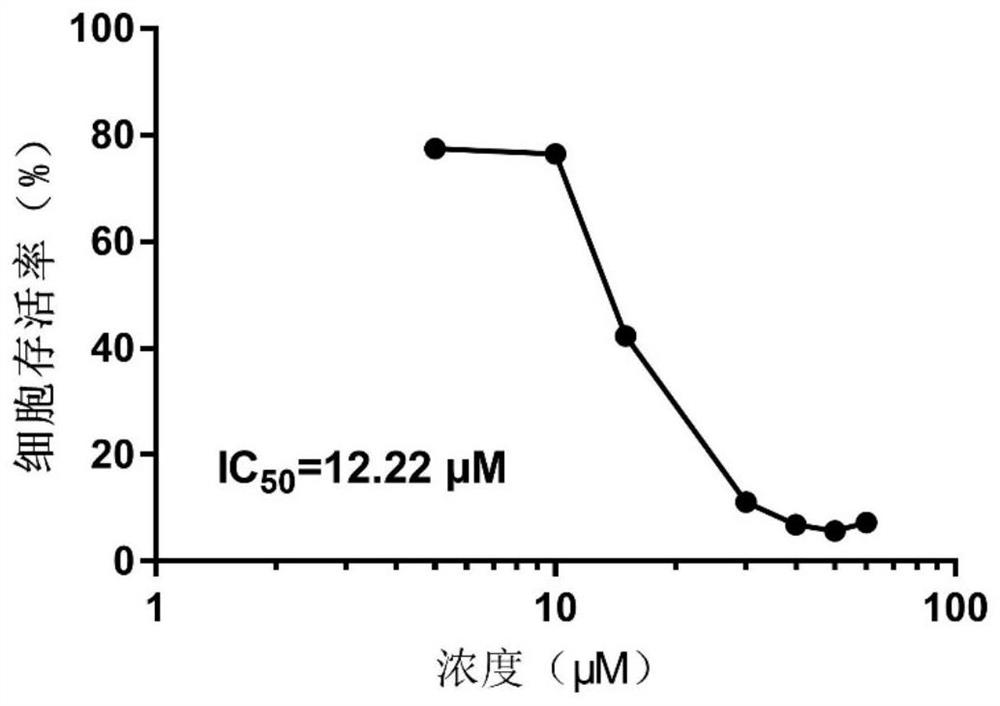
[0036] Embodiment 2, cannabidiol (CBD) is to the inhibitory effect of human acute lymphoblastic leukemia cell Reh survival in vitro
[0037] Cannabidiol (CBD) was dissolved in DMSO to a concentration of 50 μM and stored at -20°C until use. Cannabidiol (CBD) was further diluted to the required concentration before use, and the DMSO concentration was determined to be less than 0.001%. The human acute B-lymphoblastic leukemia cell line Reh used in this experiment was used as the test cell. The cells were cultured at 37°C in a humid atmosphere with 5% CO2 and 95% air, and the culture medium was RPMI-1640 containing 10% heat-inactivated fetal bovine serum. The survival of human acute B-lymphoblastic leukemia cells Reh was tested at various concentrations of cannabidiol (CBD).
[0038] result: figure 2 The survival of human acute B-lymphoblastic leukemia cells Reh in response to cannabidiol (CBD) administration is described. It can be seen that as the dose of cannabidiol (CBD) ...
Embodiment 3
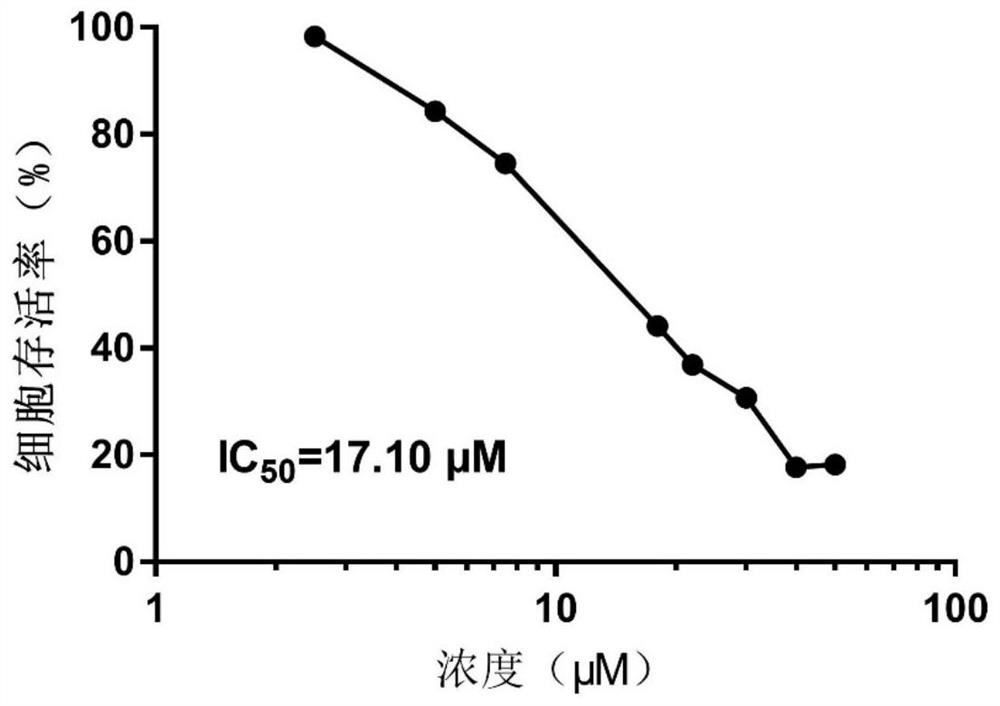
[0039] Embodiment 3, cannabidiol (CBD) is to human acute lymphoblastic leukemia cell RS4 in vitro; 11 survival inhibitory effect
[0040] Cannabidiol (CBD) was dissolved in DMSO to a concentration of 50 μM and stored at -20°C until use. Cannabidiol (CBD) was further diluted to the required concentration before use, and the DMSO concentration was determined to be less than 0.001%. The human acute lymphoblastic leukemia cell line RS4;11 used in this experiment was used as the test cell. The cells were cultured at 37°C in a humid atmosphere with 5% CO2 and 95% air, and the culture medium was RPMI-1640 containing 10% heat-inactivated fetal bovine serum. The survival of human acute B-lymphoblastic leukemia cells RS4;11 was tested at various cannabidiol (CBD) concentrations.
[0041] result: image 3 The viability of human acute B-lymphoblastic leukemia cells RS4;11 in response to cannabidiol (CBD) administration is described. It can be seen that as the dose of cannabidiol (CBD)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com