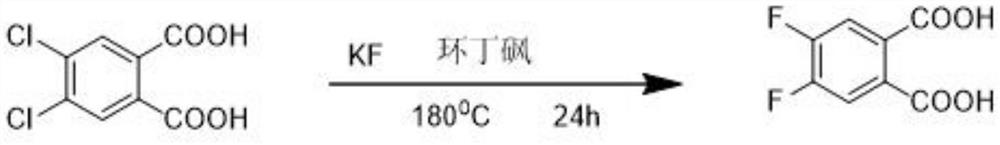
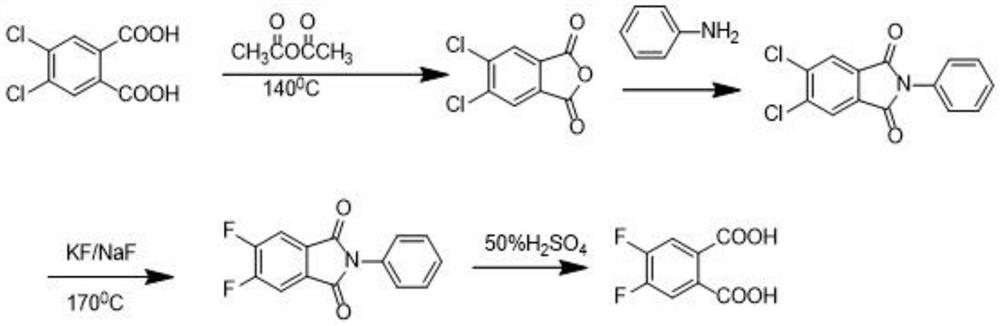
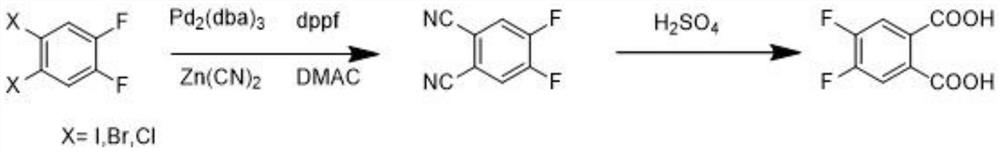
The preparation method of 4,5-difluorophthalic acid
A technology of difluorophthalic acid and phthalic acid, which is applied in the field of compound preparation, can solve problems such as difficulty in obtaining raw materials, and achieve the effects of low cost of raw materials, easy industrial production, and safe, efficient and easy-to-operate process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 4,5-difluoro-1,2-phthalonitrile: add 271.8g of 1,2-dibromo-4,5-difluorobenzene and 27g of polymethylpolysiloxane to a 3000mL reaction flask , stirred with 1450 mL of N,N-dimethylacetamide (DMAC) to dissolve, and after three nitrogen replacements, heated to 100 °C for 1 h. Turn off the heating, add 16.3 g of tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 ) and 12.3g of 1,1'-bis(diphenylphosphino)ferrocene (dppf), then slowly added 120g of zinc cyanide, reacted at 100°C for 16 hours, and monitored the reaction by thin layer chromatography (TLC). After the reaction of the raw materials was completed, the reaction was stopped and the temperature was lowered. The reaction solution was poured into 7 L of water, and 2 mol / L of sodium bicarbonate solution was slowly added under vigorous stirring to adjust the pH to about 8. Fully stirred for 3 hours, filtered, the filter cake was rinsed with ethyl acetate until there was no coloration, the filtrate was extra...
Embodiment 2
[0030] Synthesis of 4,5-difluoro-1,2-phthalonitrile: add 271.8g of 1,2-dibromo-4,5-difluorobenzene and 33.8g of polymethyl polysiloxane to a 3000mL reaction flask alkane, dissolved with 1800 mL of DMAC by stirring, replaced with nitrogen three times, heated to 100 °C, and reacted for 1 h. Turn off the heat and add 18g of Pd 2 (dba) 3 and 14.5g of dppf, slowly add 120g of zinc cyanide, react at 120°C for 10 hours, monitor the reaction by thin layer chromatography (TLC), stop the reaction after the reaction of the raw materials and cool down, pour the reaction solution into 7L of water, stir vigorously Then slowly add 2mol / L sodium bicarbonate solution to adjust the pH to about 8. Stir well for 3 hours and filter. The filter cake was rinsed with ethyl acetate until there were no spots and no color was developed, the filtrate was extracted with ethyl acetate, the combined organic phases were backwashed twice with saturated brine, and dried over anhydrous sodium sulfate. The o...
Embodiment 3
[0033] Synthesis of 4,5-difluoro-1,2-phthalonitrile: add 271.8g of 1,2-dibromo-4,5-difluorobenzene and 33.8g of polymethyl polysiloxane to a 3000mL reaction flask alkane, dissolved in 1000 mL of DMAC with stirring, replaced with nitrogen three times, heated to 100 °C, and reacted for 1 h. Turn off the heat and add 16.3 g of Pd 2 (dba) 3 and 12.3g of dppf, slowly add 120g of zinc cyanide, react at 100°C for 10 hours, monitor the reaction by thin layer chromatography (TLC), stop the reaction after the reaction of the raw materials and cool down, pour the reaction solution into 7L of water, stir vigorously Then slowly add 2mol / L sodium bicarbonate solution to adjust the pH to about 8. Stir well for 3 hours and filter. The filter cake was rinsed with ethyl acetate until there were no spots and no color was developed, the filtrate was extracted with ethyl acetate, the combined organic phases were backwashed twice with saturated brine, and dried over anhydrous sodium sulfate. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com