Method for establishing fingerprint spectrum of Draba nemorosa and Chinese date lung-purging granules
A technology of fingerprints and standard fingerprints, applied in the field of establishment of HPLC fingerprints of Tingli Dazao Xiefei Granules, to achieve the effects of many characteristic peaks, good baseline separation, and good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
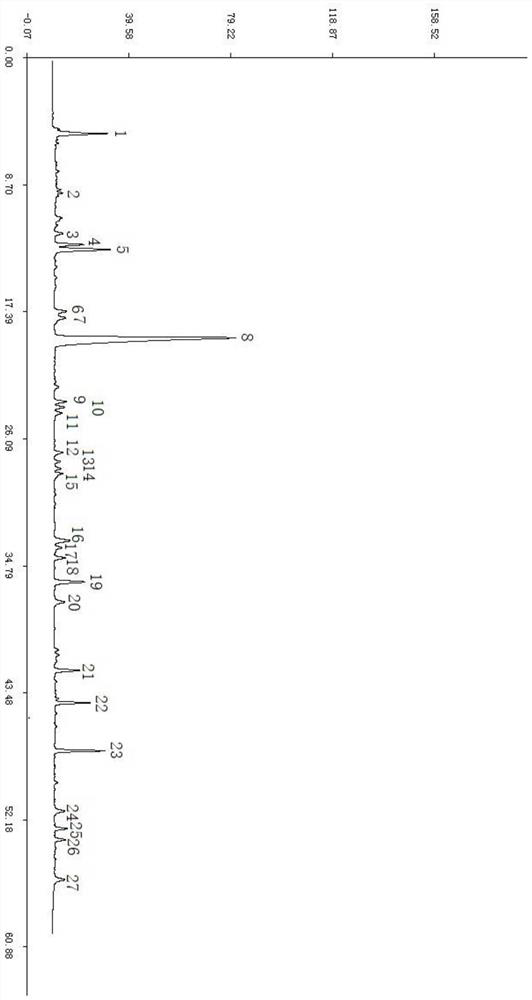
[0027] Example 1 Establishment of HPLC Standard Fingerprint of Tingli Dazao Xiefei Granules
[0028] 1 Instruments and reagents
[0029] 1.1 Instrument
[0030] Agilent 1260 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0031] 1.2 Reagent
[0032] Tingli Dazao Xiefei Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 1. Acetonitrile was chromatographically pure, water was double distilled water, and other reagents were analytically pure.
[0033] Table 1 Batch No. of Test Samples of Tingli Dazao Xiefei Granules
[0034]
[0035]
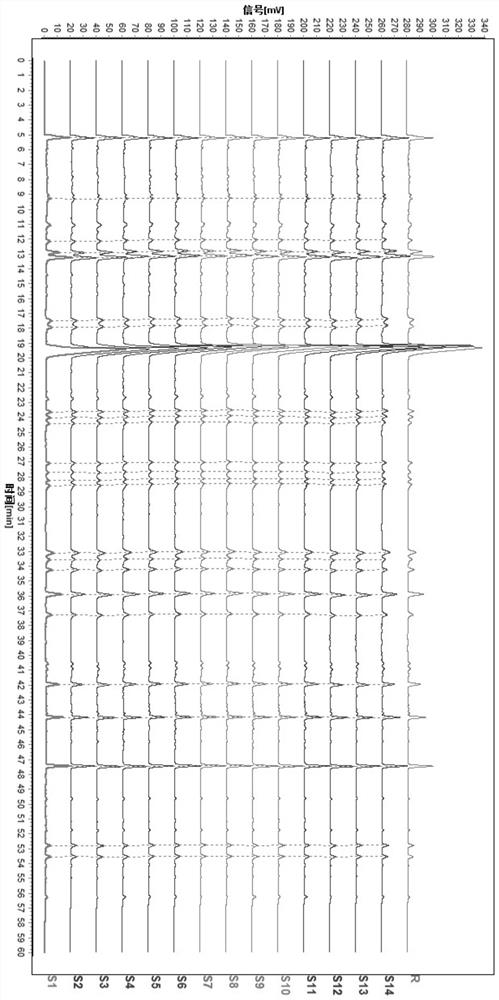
[0036] 2 Methods and Results
[0037] 2.1 Chromatographic conditions: chromatographic column: Agilent ZORBAX SB C18 (4.6×250mm, 5μm) column; mobile phase: acetonitrile as mobile phase A, 0.12% phosphoric acid aqueous solution as mobile phase B, gradient as shown in the table below ...
Embodiment 2
[0056] Example 2 Establishment of HPLC Standard Fingerprint of Tingli Dazao Xiefei Granules
[0057] 1 Instruments and reagents
[0058] 1.1 Instrument
[0059] Agilent 1100 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0060] 1.2 Reagent
[0061] Tingli Dazao Xiefei Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 4; acetonitrile was chromatographically pure, water was double distilled water, and the rest of the reagents were analytically pure.
[0062] Table 4 Batch numbers of test samples of Tingli Dazao Xiefei Granules
[0063]
[0064] 2 Methods and results
[0065] 2.1 Chromatographic conditions: Chromatographic column: Agilent ZORBAX SB C18 (4.6×250mm, 5μm) column; mobile phase: acetonitrile as mobile phase A, 0.18% by volume phosphoric acid aqueous solution as mobile phase B, gradient as shown in the ta...
Embodiment 3
[0071] Example 3 Establishment of HPLC Standard Fingerprint of Tingli Dazao Xiefei Granules
[0072] 1 Instruments and reagents
[0073] 1.1 Instrument
[0074] Agilent 1100 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0075] 1.2 Reagent
[0076] Tingli Dazao Xiefei Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 5; acetonitrile was chromatographically pure, water was double distilled water, and other reagents were analytically pure.
[0077] Table 5 Batch numbers of test samples of Tingli Dazao Xiefei Granules
[0078]
[0079] 2 Methods and Results
[0080] 2.1 Chromatographic conditions: chromatographic column: Agilent ZORBAX SB C18 (4.6×250mm, 5μm) column; mobile phase: acetonitrile as mobile phase A, 0.12% phosphoric acid aqueous solution as mobile phase B, gradient as shown in the table below Elution: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com