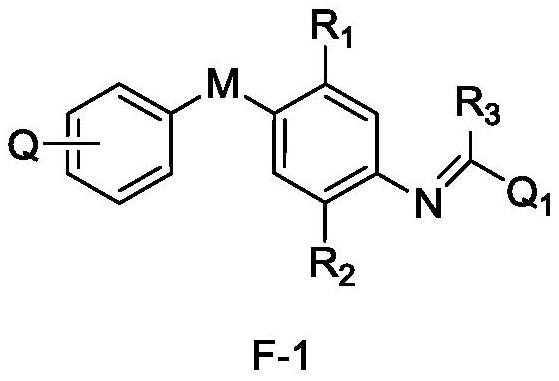
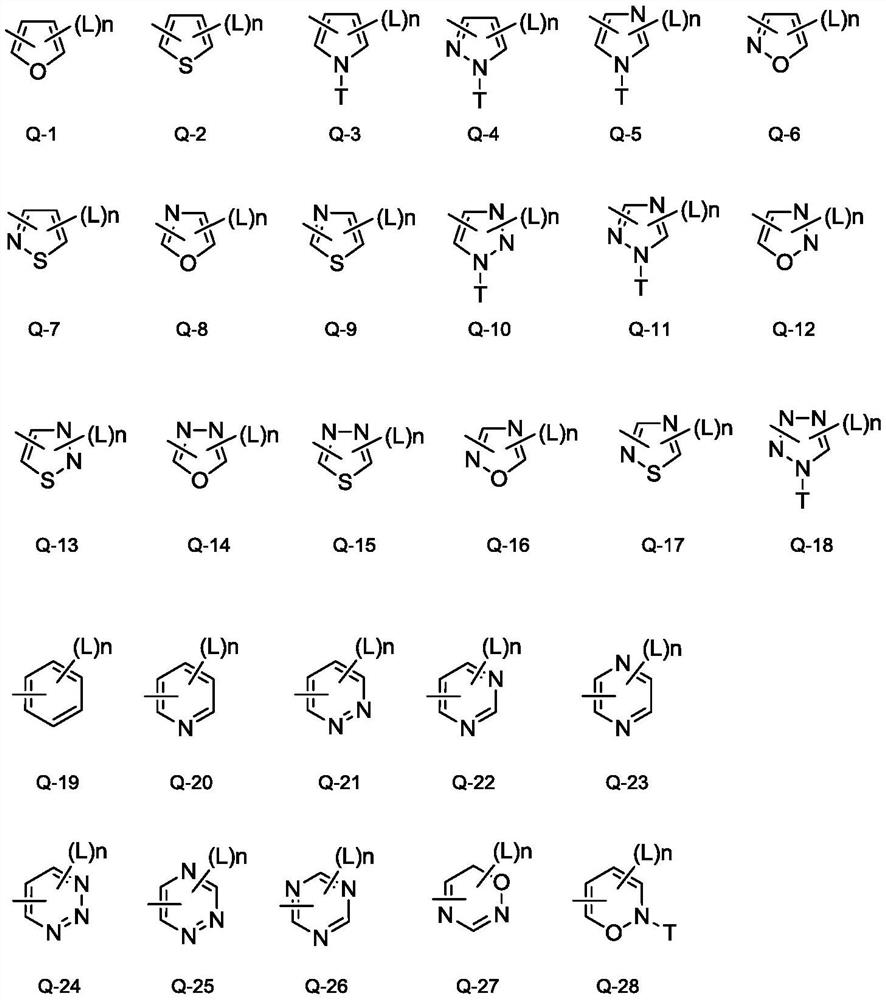
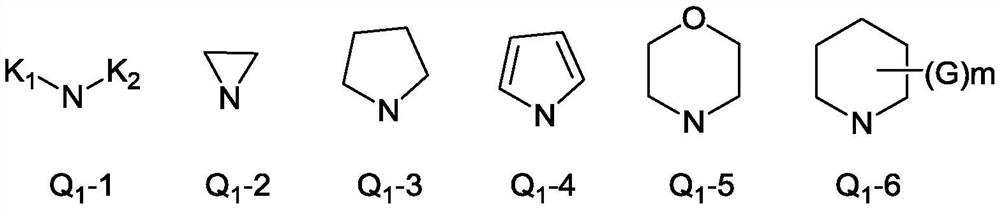
Aryl amidine structure-containing compound, preparation method and application thereof
A technology for aryl amidines and compounds is applied in the field of compounds containing aryl amidine structures, and can solve the problems of not showing advantages and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1: compound 2 is synthesized
[0103] synthetic route:
[0104]
[0105] Synthetic steps:
[0106] (1) Preparation of intermediate A3:
[0107] Add 13.7g (0.1mol) m-chlorobenzonitrile A1 and 12.2g (0.1mol) 2,5-dimethylphenol A2 to 250mL dry DMF, add 39g (0.12mol) cesium carbonate and 0.75g (0.005mol) ) sodium iodide, dry protection, warming up to reflux, TLC tracking to the end of the reaction. After the reaction was completed, it was cooled, and the reaction liquid was poured into ice water, extracted with ethyl acetate, washed with water, dried, and concentrated to obtain 20.5 g (0.092 mol) of light black liquid intermediate A3 with a yield of 92.1%.
[0108] After testing, the prepared compound is A3, and its performance data are as follows:
[0109] 1 H NMR (600MHz, CDCl 3 )δ:2.13(s,3H,CH3),2.31(s,3H,CH3),6.76(s,1H,Ar-H),6.96-6.97(d,1H,Ar-H),7.07(m,1H ,Ar-H),7.12-7.14(dd,1H,Ar-H),7.15-7.17(d,1H,Ar-H),7.29-7.31(d,1H,Ar-H),7.37-7.39(t ,1H,Ar-H); ...
Embodiment 2
[0130] Embodiment 2: Compound 8 is synthesized
[0131] Compound 8 was prepared by the same method as in Example 1. The performance data of the obtained compound 8 are as follows:
[0132] 1 H NMR (600MHz, CDCl 3 )δ:2.11(s,3H,CH3),2.22(s,3H,CH3),3.03(s,6H,(CH3)2),6.65(s,1H,Ar-H),6.79(s,1H, Ar-H),7.04-7.06(dd,1H,Ar-H),7.38-7.41(t,1H,Ar-H),7.45(s,1H,CH),7.57-7.58(m,1H,Ar- H),7.72-7.73(d,1H,Ar-H); ESI-MS m / z:405[M+H] + .
Embodiment 3
[0133] Embodiment 3: the synthesis of compound 10
[0134] Compound 10 was prepared by the same method as in Example 1. The performance data of the obtained compound 10 are as follows:
[0135] 1 H NMR (600MHz, CDCl 3 )δ:0.93-0.95(t,3H,J=7.2Hz,CH3),1.65(m,2H,CH2),2.11(s,3H,CH3),2.20(s,3H,CH3),3.01(s, 3H, CH3), 3.23(m, 2H, CH2), 6.65(s, 1H, Ar-H), 6.79(s, 1H, Ar-H), 7.04-7.06(d, 1H, J=8.3Hz, Ar -H), 7.38-7.41(t, 1H, J=8.1Hz, Ar-H), 7.45(s, 1H, CH), 7.56-7.58(m, 1H, Ar-H), 7.72-7.73(d, 1H, J=7.7Hz, Ar-H); ESI-MS m / z: 405[M+H] + ..
[0136] (2) preparation preparation
[0137] The following examples are prepared according to the mass ratio.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com