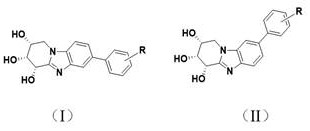
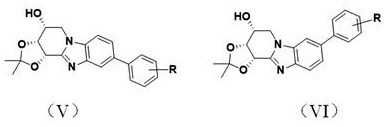
Biphenyl benzimidazoloazepine derivatives and their synthesis methods and applications
A technology of biphenyl benzimidazole and synthesis method, which is applied in the directions of drug combination, organic chemical method, bulk chemical production, etc., to achieve high efficiency of preparation method, superior β-glucosidase inhibitory activity, and strong anti-glucosidase abnormality Effects of expression-induced related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
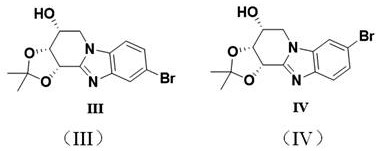
[0034] Synthesis of (3aR,4R,11bS)-9-bromo-2,2-dimethyl-3a,4,5,11b-tetrahydrobenzo[4,5]imidazol[1,2-a][1,3 ]dioxo[4,5-c]pyridin-4-ol (referred to as compound 3) and (3aR,4R,11bS)-8-bromo-2,2-dimethyl-3a,4,5,11b- Tetrahydrobenzo[4,5]imidazol[1,2-a][1,3]dioxo[4,5-c]pyridin-4-ol (Compound 4 for short)
[0035] Its chemical reaction process is as follows:
[0036]
[0037] The specific method is:
[0038] Weigh D-ribose 1 (4.3 g, 12.5 mmol, commercially available or using D-ribose as raw material, which is protected by propylidene and p-tosylated (Tsylated), refer to the literature method Aravind, A., et al. , Eur.J.Org.Chem., 2011,83,6980-6988) and 4-bromo-1,2-o-phenylenediamine (2.79g, 1.2 equivalents, commercially available) in a 250mL flask, add 80mL The toluene was stirred and dissolved, and scandium trifluoromethanesulfonate (0.62 g, 0.1 equivalent, commercially available) was weighed, and the temperature of the oil bath was raised to 80° C. under nitrogen protection. ...
Embodiment 2
[0042] Synthesis of (3aR,4R,11bS)-9-(3,4-dimethoxyphenyl)-2,2-dimethyl-3a,4,5,11b-tetrahydrobenzo[4,5]imidazole And[1,2-a][1,3]dioxo[4,5-c]pyridin-4-ol (compound 6a for short)
[0043] Its chemical reaction process is as follows:
[0044]
[0045] The specific method is:
[0046] Weigh compound 3 (0.3g, 0.9mmol) and 3,4-dimethoxyphenylboronic acid (0.19g, 1.2 equivalents, commercially available) in a 25mL flask, add water and 1,4-dioxane 7mL was used as solvent, cesium carbonate (0.87g, 3.0 equivalents) was added, vacuumed, filled with nitrogen, and then pd(ppf)Cl was added 2 (0.07g, 0.1 equivalent, commercially available), vacuumize, fill with nitrogen, heat up to 95°C for reaction, TLC monitors that the reaction of raw material compound 3 is complete, extract with water and ethyl acetate, take the organic phase, dry over anhydrous sodium sulfate, reduce Pressure evaporated to remove solvent, 300 mesh silica gel column chromatography (V 二氯甲烷 :V 乙酸乙酯 =2:1), the product...
Embodiment 3
[0049] Synthesis of (2R,3R,4S)-7-(3,4-dimethoxyphenyl)-1,2,3,4-tetrahydrobenzo[4,5]imidazo[1,2-a] Pyridine-2,3,4-triol (compound 7a for short)
[0050] Its chemical reaction process is as follows:
[0051]
[0052] The specific method is:
[0053] Weigh compound 6a (0.26 g, 0.66 mmol) into a 25 mL flask, add 4 mL of 30% trifluoroacetic acid aqueous solution to dissolve. The reaction was carried out at room temperature under the protection of nitrogen. After 5 hours of reaction, TLC monitored that the reaction of the raw materials was complete. Sodium bicarbonate neutralizes the reaction solution, removes the salt by suction filtration, evaporates the solvent under reduced pressure from the filtrate, and separates by chromatography on a 200-mesh silica gel column (V 二氯甲烷 :V 甲醇 =15:1), a white solid 7a was obtained.
[0054] Compound 7a: white solid, yield 50%. (c 1mg / mL, CH 3 OH). 1 H NMR (400MHz, DMSO-d 6 )δ(ppm):7.82-7.75(m,1H),7.61(dd,J=8.4,2.0Hz,1H),7.52-7.47(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com