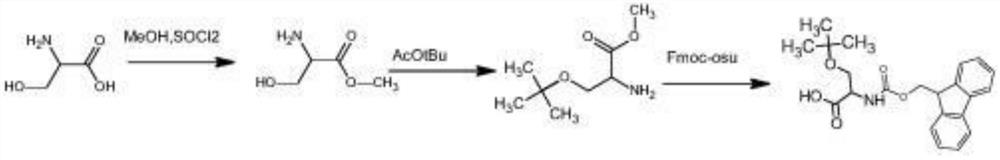
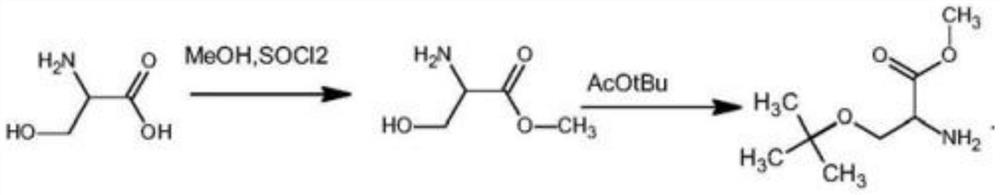
Preparation method of O-tert-butyl-L-serine methyl ester and O-tert-butyl-L-serine aqueous solution
A technology of serine methyl ester and tert-butyl, which is applied in the field of medicinal chemistry and can solve the problems of easy racemization in the synthesis process, difficult control of process parameters, and easy production of more impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 20g of L-serine and 600g of methanol into the reaction flask, and add 28g of SOCl dropwise to the reaction flask under stirring 2 , use reflux reaction, and use TLC to detect the reaction solution in the reaction bottle. If there is no L-serine in the reaction bottle, the reaction is complete; The solution obtained L-serine methyl ester hydrochloride solid 29g, wherein the yield of L-serine methyl ester hydrochloride was 98%;
[0048] Get a new reaction flask, add 410g tert-butyl acetate, 50g perchloric acid (analytical pure) and 29g L-serine methyl ester hydrochloride successively in this reaction flask, then add 10g H 2 SO 4 (analytically pure), at room temperature, stirred and reacted for 3 days, when TLC detection (thin-layer chromatography) detected that there was substantially no L-serine methyl ester hydrochloride in the reaction system, 50g of water was added to the reaction bottle, and the reaction was adjusted with NaOH solution The PH in the bottle is 8...
Embodiment 2
[0053] Add 20g of L-serine and 600g of methanol into the reaction flask, and add 30g of SOCl dropwise to the reaction flask under stirring 2 , use reflux reaction, and use TLC to detect the reaction solution in the reaction bottle. If there is no L-serine in the reaction bottle, the reaction is complete; The solution obtained L-serine methyl ester hydrochloride solid 29g, wherein the yield of L-serine methyl ester hydrochloride was 98%;
[0054] Get a new reaction bottle, add 410g tert-butyl acetate, 52g perchloric acid (analytical pure) and 29g L-serine methyl ester hydrochloride successively in this reaction bottle, then add 12g H 2 SO 4 (analytical pure), at room temperature, stirred and reacted for 4 days, when TLC detection (thin layer chromatography) detects that there is substantially no L-serine methyl ester hydrochloride in the reaction system, add 60g water in the reaction flask, adjust the reaction with NaOH solution The pH in the bottle is 9, let stand to separat...
Embodiment 3
[0059] Add L-serine 10g, methyl alcohol 300g in the reaction bottle, under stirring condition, in the reaction bottle, drip the SOCl of 14g , adopt reflux reaction, and detect the reaction solution in the reaction bottle with TLC detection, there is no in the reaction bottle To L-serine, the reaction is complete; at 40 ° C, the reaction solution in the reaction bottle is concentrated under negative pressure, and the solution in the reaction solution is removed to obtain 15 g of L-serine methyl ester hydrochloride solid, of which L-serine methyl ester salt The yield of acid salt is 98.6%;
[0060] Get a new reaction flask, add about 210g tert-butyl acetate, 25g perchloric acid (analytical pure), 15g L-serine methyl ester hydrochloride successively in this reaction flask, then add 5g H 2 SO 4 (analytically pure), at room temperature, stirred and reacted for 4 days, when TLC detection (thin layer chromatography) detects that there is substantially no L-serine methyl ester hydroc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com